In the field of life sciences, the sugar chains on the surface of cells act as complex “molecular codes” that carry critical biological information. Among them, sialic acid, a sugar molecule with a nine-carbon backbone, is typically located at the terminal of cell surface sugar chains and plays an important role in various biological processes such as signal transduction and immune response. Notably, cancer cells overexpress sialic acid on their surface to evade recognition by the immune system and promote metastasis, using it as a “biological mask,” making sialic acid a highly promising biomarker for cancer diagnosis. However, traditional methods for detecting sialic acid, such as high-performance liquid chromatography, mass spectrometry, and colorimetric methods, have limitations in providing dynamic in vivo information, making it difficult to meet the real-time monitoring needs of cancer diagnosis. Recent sensing strategies also face challenges such as high costs, low selectivity, or difficulties in synthesis. Therefore, developing an efficient method for real-time visualization of sialic acid in vivo has become a pressing scientific issue in this field.
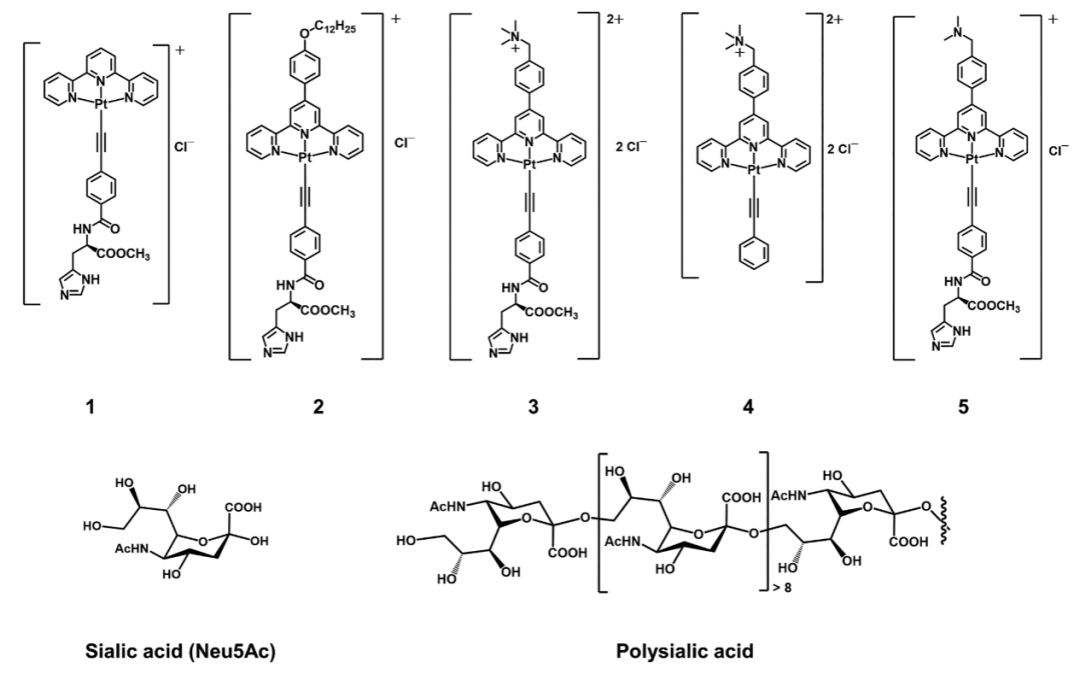
Scientists have turned their attention to the self-assembly characteristics of alkynyl platinum(II) complexes. These complexes possess a unique square planar geometry that allows for self-assembly through Pt(II)…Pt(II) interactions and π-π stacking. Researchers introduced histidine moieties and positively charged trimethylammonium groups into the complexes to construct a sophisticated molecular recognition system. The histidine moiety forms specific hydrogen bonds with sialic acid, while the positively charged trimethylammonium group interacts electrostatically with the negatively charged carboxyl groups of sialic acid. These two interactions synergistically enhance the binding affinity of the complex to sialic acid. When the complex binds to sialic acid, it triggers supramolecular self-assembly, causing the originally dispersed molecules to aggregate into nanofiber structures. This self-assembly process is accompanied by significant changes in luminescent properties, with a strong emission signal appearing in the near-infrared region, due to the formation of a metal-to-ligand charge transfer (MLCT) excited state during the self-assembly process, thus achieving sensing and visualization of sialic acid.
In terms of solution characterization, various techniques provided strong evidence for the interaction between the complex and sialic acid. UV-visible spectroscopy showed that upon the addition of poly-sialic acid, the low-energy absorption band of the complex at 445 nm decreased and slightly red-shifted, while an absorption shoulder appeared at 600 nm, indicating the formation of metal-metal interactions. In fluorescence titration experiments, the emission intensity in the near-infrared region (around 760 nm) significantly increased with the concentration of poly-sialic acid, further confirming the luminescent changes induced by self-assembly. Transmission electron microscopy (TEM) images visually demonstrated the morphological transformation of the complex before and after binding to sialic acid, from a dispersed state to aggregated nanofibers with a width of approximately 20-60 nm. Dynamic light scattering (DLS) data showed that the average hydrodynamic diameter of the complex increased from 410 nm to 868 nm, confirming the formation of supramolecular assemblies. Hill coefficient analysis indicated that the binding of some complexes to poly-sialic acid exhibited a positive cooperative effect, with the binding constant (log K) of complex 3 reaching as high as 8.02 and the dissociation constant (Kd) as low as 9.52×10⁻⁹ M, demonstrating a strong binding capability.
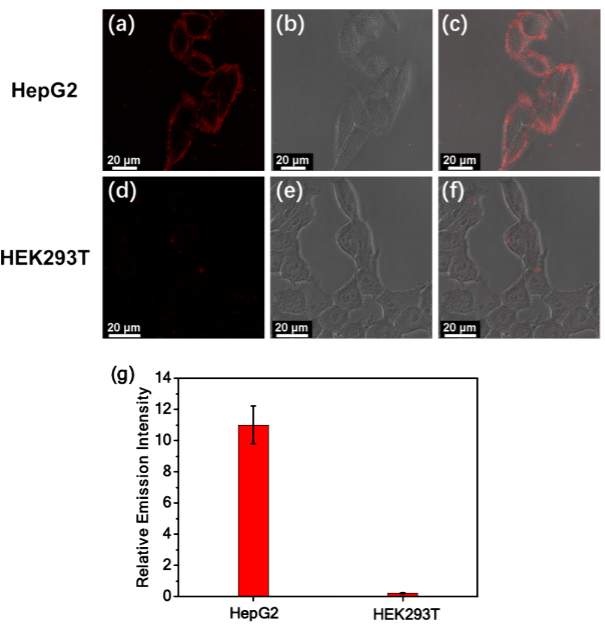
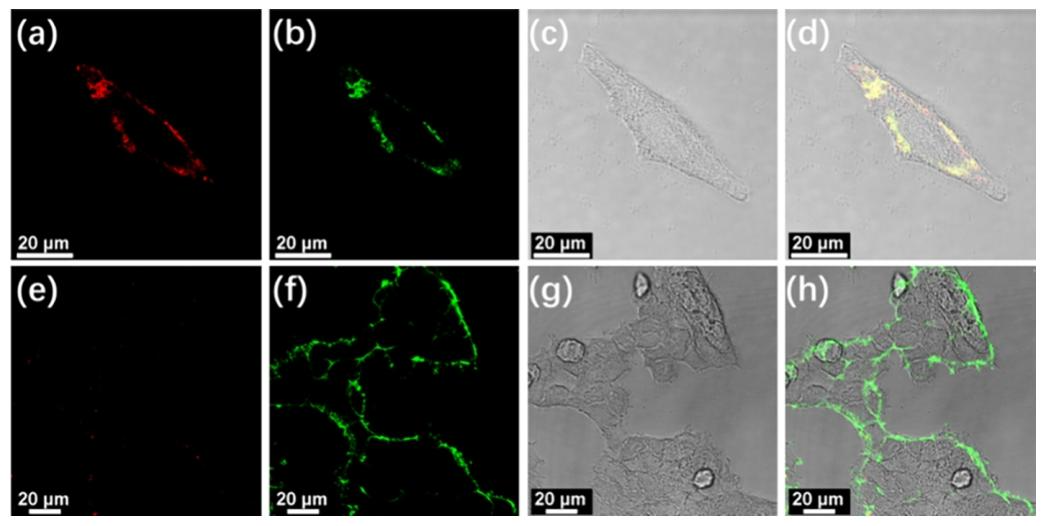
At the cellular and biological evaluation level, this system exhibited excellent cancer cell differentiation capability. In confocal imaging experiments, complex 3 produced a significant luminescent signal on the surface of liver cancer cells (HepG2) and cervical cancer cells (HeLa), while there was almost no signal on the surface of normal cells (HEK293T). This is because the high concentration of sialic acid on the surface of cancer cells promotes the luminescence of the self-assembled complex, while the low sialic acid content on normal cells cannot trigger effective assembly. Compared to commercially available dyes like FITC-labeled lectins, complex 3 has a lower false positive rate, and its unique “assembly-luminescence” response pattern avoids misjudgments that may arise from high sialic acid expression in normal cells. Additionally, complex 3 can also be used for screening sialic acid inhibitors. After treatment with neuraminidase, the sialic acid levels on the surface of cancer cells decreased, and the luminescent signal of complex 3 weakened, providing a new tool for anticancer drug development. Cytotoxicity experiments showed that complex 3 has good biocompatibility at low concentrations, with cell viability remaining above 70% after 48 hours of incubation, laying a safe foundation for its biomedical applications.
This study established a new system for sialic acid detection based on the self-assembly of alkynyl platinum(II) complexes, achieving high selectivity recognition and near-infrared luminescent response through the synergistic effect of histidine and trimethylammonium groups. From spectral characterization in solution to imaging applications at the cellular level, this system demonstrates potential for translation from basic research to clinical applications. Its innovation lies in the clever combination of supramolecular self-assembly and biosensing, utilizing the differences in sialic acid levels on the surfaces of cancer and normal cells to achieve precise differentiation through the presence or absence of luminescent signals, providing new ideas for early cancer diagnosis and treatment guidance. In the future, this strategy is expected to further develop into a universal platform for real-time monitoring of tumor microenvironments and screening new anticancer drugs, contributing to the advancement of precision oncology.
Original link:
https://doi.org/10.1021/jacs.5c03210
 Share
Share Save
Save View
View Like
Like