Remember to star the public account ⭐️ to see the push notifications in time.

Source: Content from scitechdaily, thank you.
Silicon and oxygen atoms were once thought to be merely insulators, but the change in the angle between silicon and oxygen atoms has opened a pathway for charge flow.
A groundbreaking discovery from the University of Michigan indicates that a new type of silicone resin can act as a semiconductor. This finding challenges the long-held belief that silicone resins are merely insulating materials.
“This material opens up opportunities for new types of flat-panel displays, flexible photovoltaic cells, wearable sensors, and even clothing that can display different patterns or images,” said Richard Laine, a professor of materials science and engineering and macromolecular science and engineering at the University of Michigan, and corresponding author of the research recently published in Macromolecular Rapid Communications.
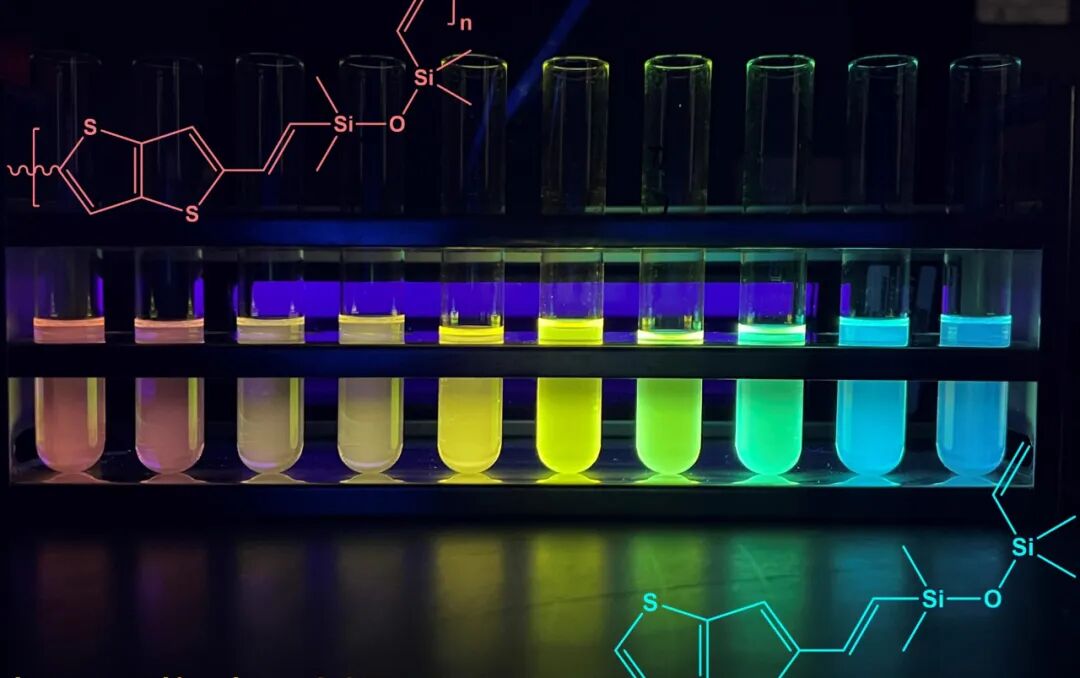
Silicone oils and silicone rubbers, also known as polysiloxanes and silsesquioxanes, have been widely used for decades due to their current and thermal conductivity properties. Their waterproof characteristics make them ideal for biomedical devices, sealants, electronic coatings, and more.
Meanwhile, traditional semiconductors are typically rigid. Silicone semiconductors have the potential to realize the flexible electronic products described by Laine and can also exhibit multiple colors.

Discovery of Molecular Structure and Conductivity
At the molecular level, organosilicon consists of a framework of alternating silicon and oxygen atoms (Si—O—Si), with organic (carbon-based) groups attached to the silicon atoms. When polymer chains are interconnected (i.e., cross-linked), various three-dimensional structures are formed, altering the physical properties of the material such as strength or solubility.
While studying different cross-linked structures of organosilicon, the research team accidentally discovered the conductive potential of copolymers, which are polymer chains containing two different types of repeating units—in this case, cage-like organosilicon and linear organosilicon.
The possibility of conductivity arises from the way electrons can traverse the overlapping orbitals of the Si—O—Si bonds. Semiconductors primarily exist in two states: the non-conductive ground state and the conductive excited state. The excited state occurs when some electrons transition to the next electron orbital, which is connected to the material (e.g., metal).
Typically, the Si—O—Si bond angle cannot achieve this connection. The 110° bond angle is far from the 180° linearity. However, the team found that in silicone copolymers, these bonds start at 140° in the ground state and extend to 150° in the excited state. This is sufficient to create a pathway for charge flow.
“This leads to unexpected interactions between electrons across multiple bonds, including the Si—O—Si bonds in these copolymers,” Laine said. “The longer the chain, the easier it is for electrons to travel longer distances, thereby reducing the energy required to absorb light and emit light at lower energy.”

Chromatography and Chain Length Control
The semiconductor properties of silicone copolymers also give them a rich color spectrum. Electrons transition between the ground and excited states by absorbing and emitting photons (light particles). The emission of light depends on the length of the copolymer chains, which Laine’s team can control. The longer the chain, the smaller the transition, and the lower the photon energy, resulting in silicone appearing red. Conversely, shorter chains require greater energy for electron transitions, thus emitting higher energy blue light, leaning towards the blue end of the spectrum.
To demonstrate the connection between chain length and light absorption and emission, the researchers separated copolymers of different chain lengths and arranged them from long to short in test tubes. When exposed to ultraviolet light, each test tube absorbed and emitted different light energies, forming a complete rainbow.
The color array based on copolymer chain length is particularly unique, as silicones have so far only been considered transparent or white due to their insulating properties, which prevent them from absorbing much light.
“We took a material that everyone thought was electrically inert and gave it new life—this material can power the next generation of soft, flexible electronic products,” said Zijing Zhang (Jackie), a PhD student in materials science and engineering at the University of Michigan and the lead author of the study.
References: Zijing Zhang, Cecilia Pilon, Hana Kaehr, Pimjai Pimbaotham, Siriporn Jungsuttiwong, and Richard M. Laine, “σ–σ* Conjugation on Si─O─Si Bonds,” Macromolecular Rapid Communications, March 12, 2025. DOI: 10.1002/marc.202500081
Reference Link
https://scitechdaily.com/new-material-breaks-the-rules-scientists-turn-insulator-into-a-semiconductor/
*Disclaimer: This article is original by the author. The content reflects the author’s personal views, and Semiconductor Industry Observation reprints it only to convey a different perspective, not representing Semiconductor Industry Observation’s endorsement or support of this view. If there are any objections, please contact Semiconductor Industry Observation.
END
This is the 4072nd issue shared by the Semiconductor Industry Observation, welcome to follow.
Recommended Reading
★A Chip That Changed the World
★U.S. Secretary of Commerce: Huawei’s Chips Are Not That Advanced
★“ASML’s New Lithography Machine, Too Expensive!”
★Quietly Rising New Competitors to NVIDIA
★Chip Crash, All Blame Trump
★New Solutions Announced to Replace EUV Lithography!
★Semiconductor Equipment Giants, Salaries Soar by 40%
★Foreign Media: The U.S. Will Propose Banning Software and Hardware Made in China for Cars

Star the public account ⭐️ to see the push notifications in time, the small account prevents loss.

Seeking likes

Seeking shares

Seeking recommendations
