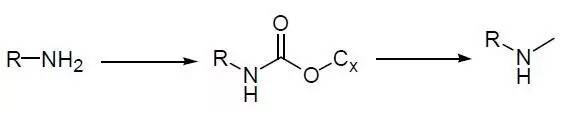
For detailed mechanisms, see: Common Reducing Agents —- Lithium Aluminum Hydride
Reaction Mechanism
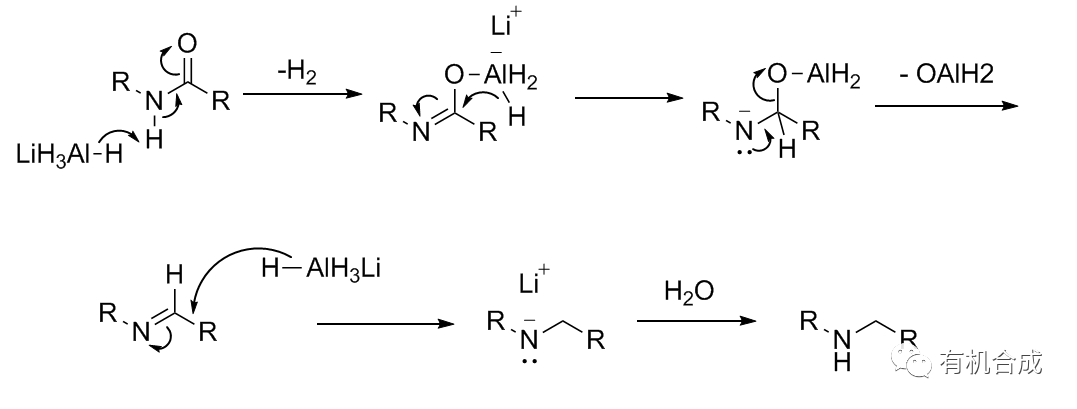
Reaction Examples1. LAH Reduction of Amides Example
LAH reduction of amides is generally performed under heated reflux, but it can also be done at room temperature without reflux; the reaction conditions are somewhat determined by the steric hindrance of the substrate. Below is a reaction example.
200 mL of anhydrous ether and 10.7 g (0.282 mol) of lithium aluminum hydride were placed in a 500 mL three-necked flask with stirrer, two-neck attachment, dropper funnel, and reflux condenser with a calcium chloride tube. A solution of 43.0 g (0.189 mol) of the starting material in 60 mL of absolute ether was added dropwise with constant stirring to maintain the ether at a steady boil. After the end of the dropwise addition, the mixture was stirred for a further 6 hours while simultaneously being refluxed. The excess lithium aluminum hydride was then decomposed by the dropwise addition of 11 mL of water, 11 mL of 15% aqueous sodium hydroxide solution, and 33 mL of water, one after the other, and the precipitated aluminum oxide hydrate was filtered off. The filtrate was dried over sodium sulfate, the solvent was removed, and the residue was distilled in a fine vacuum. 12.7 g of a colorless oil were obtained.
2. Borane-Dimethyl Sulfide Reduction of Amides Example
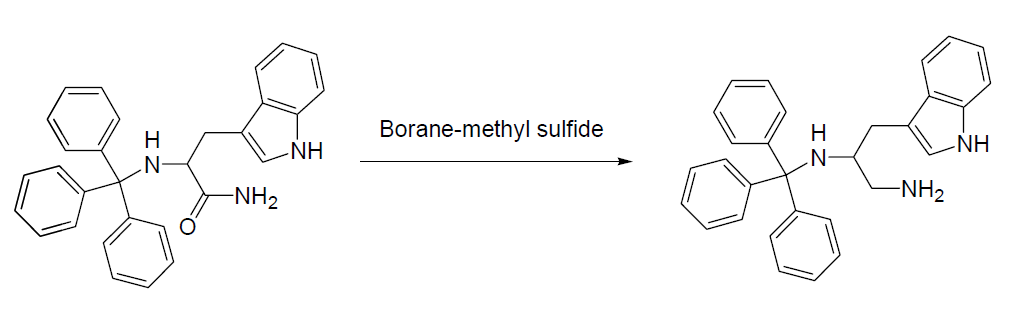
Under argon, the starting material (48.46 g, 0.108 mol) was suspended in dry tetrahydrofuran (270 mL). This mixture was then heated to reflux. Borane-methyl sulfide complex (41.3 g, 0.543 mol) was then slowly added to the reaction mixture. All of the starting amide dissolved during the addition of the borane-methyl sulfide complex. This solution was then stirred overnight in an 83°C oil bath. After cooling, a 1:1 mixture of tetrahydrofuran: water (75 mL total) was then added to the solution. Sodium hydroxide (5N, 230 mL) was then added to the mixture, which was then heated to reflux for about 30 minutes. After partitioning the aqueous and organic layers, the organic layer was collected. The aqueous layer was then extracted with tetrahydrofuran. The organic layers were combined, and the solvents were then removed by evaporation. The resulting liquid was then partitioned between ethyl acetate and brine and was washed a second time with brine. The solution was then dried over sodium sulfate, and the solvents were removed in vacuo to yield 46.68 g of the desired product.
3. NaBH4-Lewis Acid System Reduction of Amides
3.1 NaBH4-BF3 System Reduction of Amides
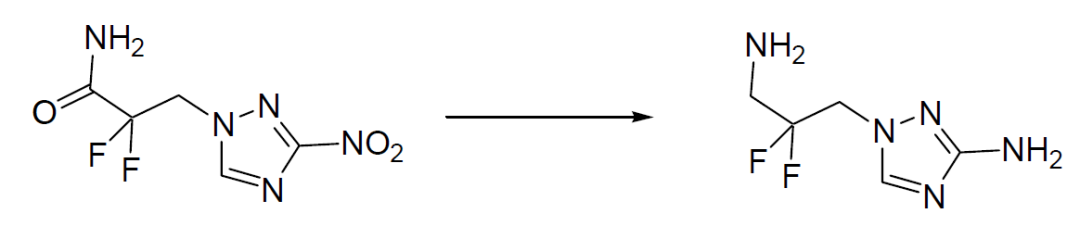
To a solution of 8.8 g (39.8 mmol) of the starting material in 100 mL of dry THF, 4.52 g (119 mmol) of sodium borohydride was slowly added with cooling by ice under nitrogen atmosphere. To the obtained suspension, a solution of 16.1 mL of BF3-ether complex in 50 mL of THF was slowly added with cooling by ice and then stirred for 4 hours with cooling by ice and for 4 hours at room temperature. Then, the reaction solution was poured into water, acidified by the addition of dilute hydrochloric acid, and stirred for two hours. The solution was alkalized by the addition of an aqueous potassium hydroxide solution and extracted with ethyl acetate. The extract was dried on magnesium sulfate and filtered. The filtrate was concentrated and subjected to purification by silica gel column chromatography to give 2.2 g of the desired product.
3.2 NaBH4-AlCl3 System Reduction of Amides
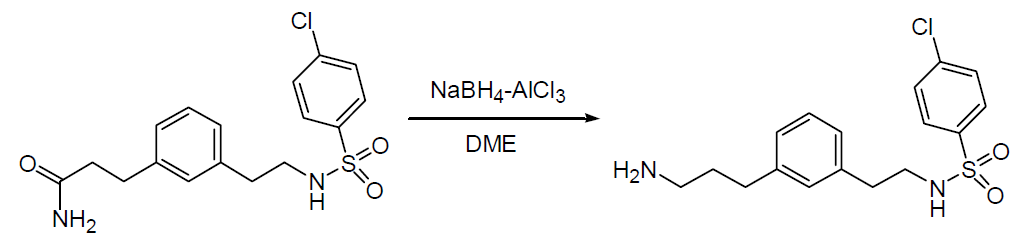
To a solution of the starting material (21.6 g, 58.6 mmol) and dimethoxyethane (175 mL), anhydrous aluminium chloride (31.3 g, 0.234 mol) was added portionwise at 15°C~20°C and allowed to stir further for 30 min. NaBH4 (8.85 g, 0.234 mol) was then added portionwise at 15°C~20°C within 1/2 hr. After 3 hrs of stirring while cooling, ice-water (130 mL) was carefully added dropwise, ensuring that the internal temperature did not exceed 25°C. While cooling with ice, the pH value was adjusted to 8.5 by the addition of concentrated NaOH, followed by the addition of 75 mL ethyl acetate and then filtered. The precipitate was washed with ethyl acetate. After separation of the phases, the aqueous phase was also extracted with ethyl acetate, and the organic extracts were dried, evaporated, and combined with the precipitate to obtain the desired product.
4. DIBAL Reduction of Amides 【Common Reducing Agent —- DIBAL】
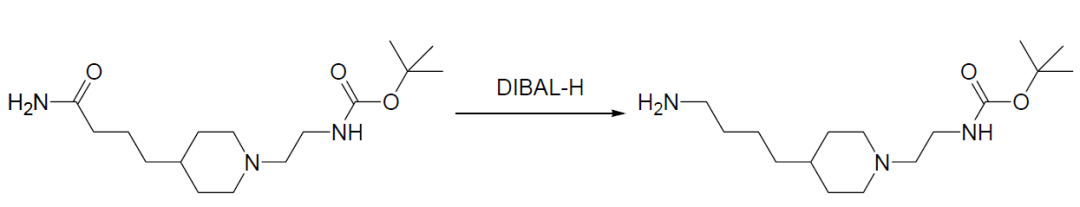
A solution of the material (250 mg, 0.80 mmol) in dichloromethane (10 mL) was cooled to 0°C, then DIBAL-H (7.4 mL, 7.4 mmol of 1M in toluene) was added dropwise into the solution over 45 min. The mixture was stirred for 1 hour, then warmed to room temperature and stirred for 14 h. The reaction was quenched with potassium sodium tartrate aqueous solution. The mixture was extracted with dichloromethane (3×10 mL). The combined extracts were washed with water and brine, dried over sodium sulfate, and concentrated under vacuum to afford an oil. Purification by column chromatography (silica; 90: 10, v/v, dichloromethane/methanol followed by 89: 10: 1 dichloromethane/methanol/ammonium hydroxide) produced the desired product (54 mg, 23% un-optimized yield) as a clear colorless oil.
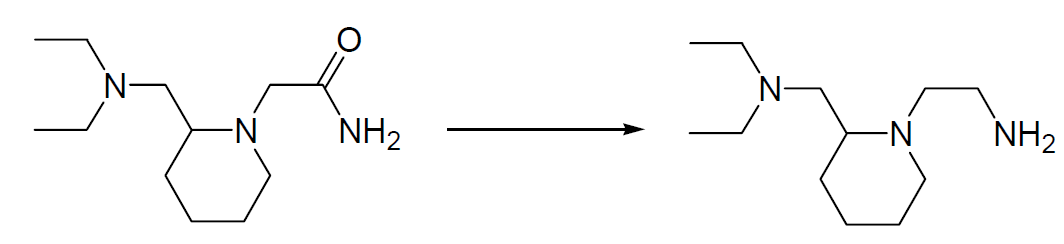
5. Primary Amines Monomethylation After Carbon Amide Reduction Example 【Common Reducing Agent —- Lithium Aluminum Hydride】
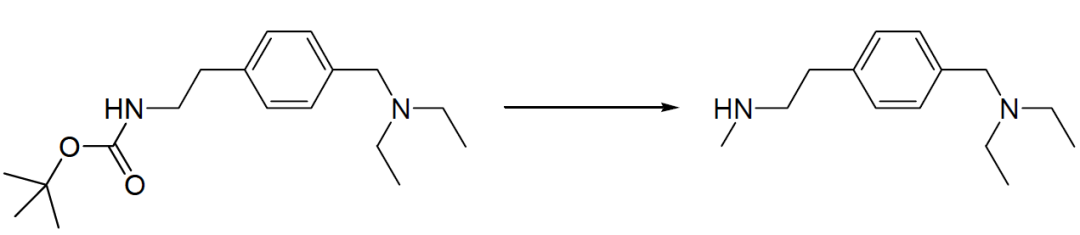
600 mg (1.96 mmol) of the material in THF is slowly added dropwise to a suspension of 250 mg (6.59 mmol) of lithium aluminum hydride in 10 mL tetrahydrofuran. The reaction is stirred overnight and heated to 50°C for a further hour. Working up is carried out by the successive addition of 0.25 mL water, 0.25 mL 15% NaOH solution, and 0.75 mL water. After filtration, the organic phase is dried over magnesium sulfate, and the solvent is eliminated using the rotary evaporator. 350 mg of the product was obtained (81.1% yield).
This content is sourced from the internet, and copyright belongs to the original author.