1. When Aniline Meets“Molecular Surgery”: An Innovative Path to Break Aromaticity
In the world of organic synthesis, aniline acts like a “stubborn guardian” — the carbon-nitrogen bond (C-N) on its aromatic ring is highly stable and difficult to modify directly. However, chiral α-branched benzylamines, as core scaffolds for drug molecules (such as analgesics and antibiotics) and natural products, have been a target for efficient synthesis pursued by chemists.
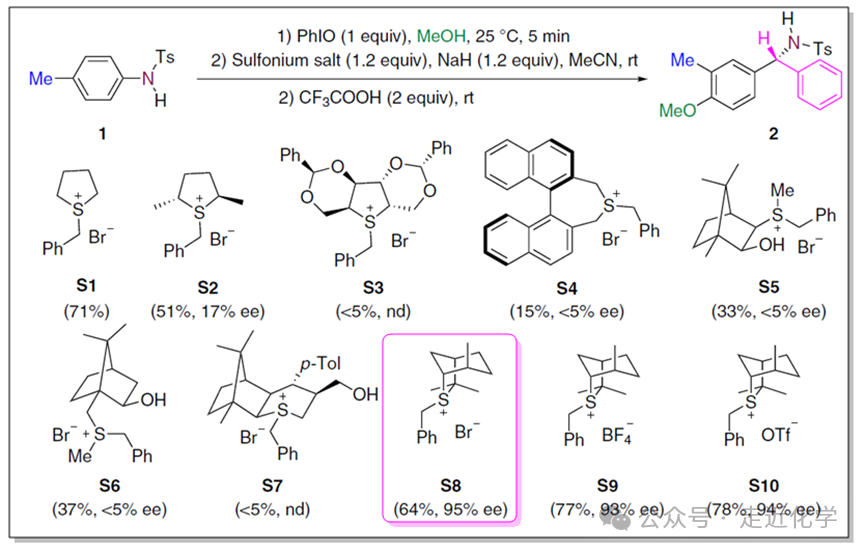
The team led by Fan Renhua at Fudan University has, for the first time, achieved a“deconstruction-reconstruction” strategy to realize theC-N bond of aniline in a“one-carbon insertion” transformation. This is akin to performing a precise“minimally invasive surgery” on the molecule: first breaking the stable structure of the aromatic ring, inserting a carbon atom unit, and then reconstructing the aromaticity, ultimately yielding high-value chiral benzylamines.[Nature Communications., DOI: 10.1038/s41467-020-18593-4]

2. Three-Step Reaction“Combination Punch”: The Comeback from Aniline to Chiral Benzylamine
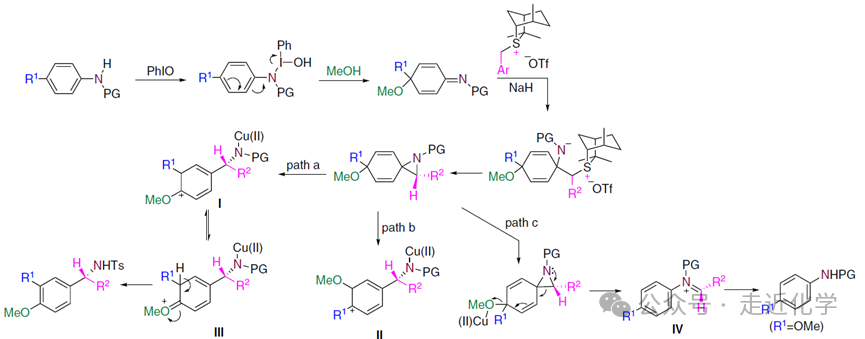
1. Oxidative De-aromatization: Breaking the Aromatic Ring
In traditional methods, theC-N bond of aniline is like a“fortress”, but the research team discovered that treating substituted anilines withPhIO (iodobenzene) in methanol can unlock the aromatic ring through an oxidative reaction, generating a cyclohexadienyl imine intermediate. This step is like tearing down the“stable walls” of the aromatic ring, allowing theC-N bond to transition from“inert” to“active.”
2. Chiral Sulfide Ylide Appears: Precise Insertion of a Carbon Unit
The key player — chiral sulfide ylide makes its appearance here. It acts like a courier carrying a“carbon unit” and undergoes an asymmetric nitrogen-containing cyclopropanation reaction with the de-aromatized intermediate under basic conditions with NaH. Among them, the sulfide ylide derived from isothiocyanatesS8 performs best, achieving a reaction yield of64% and an enantiomeric selectivity of95% (ee value). This step precisely inserts a carbon atom into the molecule while constructing a chiral center.
3. Acid-Catalyzed Rearrangement: Reconstructing Aromaticity and Completing Migration
In the final step, under the Lewis acid catalysis ofCu(OTf)₂, the molecule undergoes rearrangement: the original substituent migrates from the para position to the meta position, while the methoxy group in methanol substitutes at the para position, and the aromatic ring is reformed. This is like“rebuilding the walls”, but the structure has been completely renewed — the final product is an α-branched benzylamine with a chiral center, achieving a maximum yield of79% and maintaining anee value of 96%.
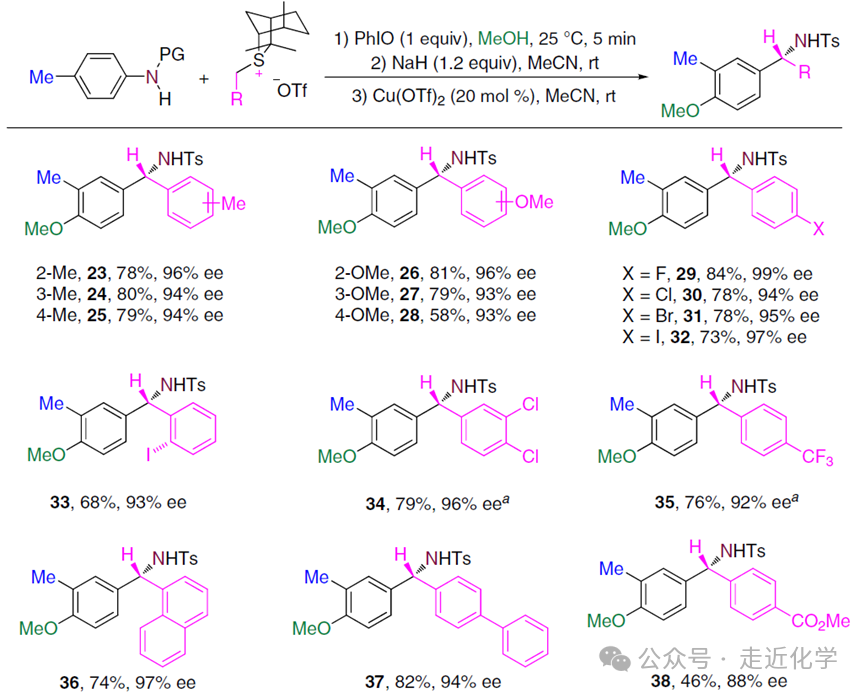
3. Substrate Expansion:“Compatibility” as a Powerful Synthetic Tool
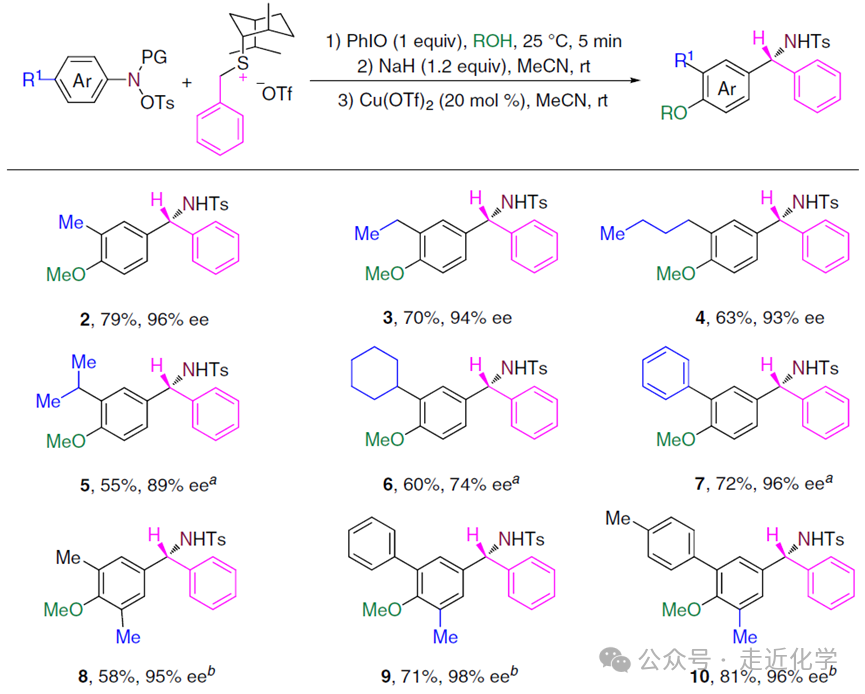
The“broad-spectrum” nature of this reaction is surprising:
- Aniline Substrates: Para substituents can be methyl, ethyl, cyclohexyl, or even phenyl, and meta and ortho substituents are also compatible. For example, ortho-fluoro-substituted aniline specifically generates migration products (ee 99%), while ortho bulky substituents (such as bromo or phenylacetylene) lead to reduced selectivity in migration position.
- Sulfide Ylide: Functional groups such as methyl, trifluoromethyl, methoxy, halogens on the aryl group, and even furan and allyl groups do not affect the reaction. For instance, 4-trifluoromethyl-substituted sulfide ylide still provides76% yield and92% ee value.
- Functional Group Introduction: By changing the solvent (such as ethanol or trifluoroethanol), ethoxy, trifluoroethoxy, and other groups can be directly introduced into the product, eliminating the need for additional modification steps.
4. Mechanism and Applications: From“Why” to“How to Do It”

-
Key Mechanism Verification
- The research team isolated intermediates48 (de-aromatized intermediate) and49 (nitrogen-containing cyclopropane intermediate), confirming that both can convert to the target product under standard conditions.
- Isotope labeling experiments indicate that the migration of substituents is aintramolecular rearrangement process rather than random recombination.
-
Synthetic Value Highlighted
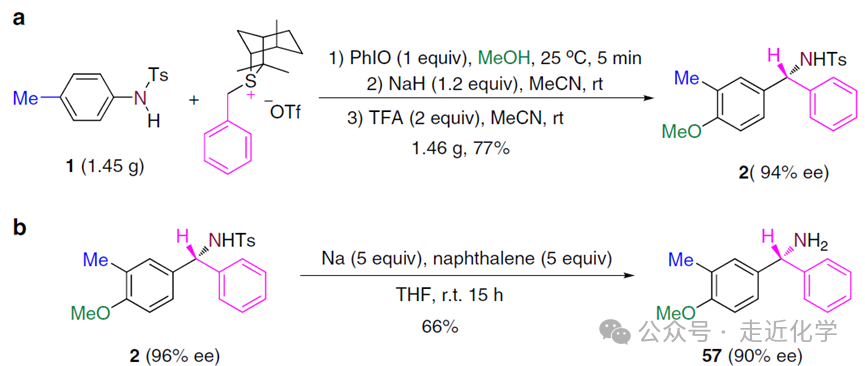
- Gram-Scale Validation: After scaling up the reaction by50 times, it still achieves77% yield and94% ee for the target product, demonstrating industrial potential.
- Derivatization: The N-Tos protecting group in the product can be reductively removed to generate free amines; iodine-substituted products can be cyclized to synthesize indole derivatives via Pd catalysis, with complete retention of chiral purity.
5. Breakthroughs and Prospects: A New Paradigm for Activating Aromatic C-N Bonds
In traditional methods, the transformation of aromaticC-N bonds often requires the nitrogen atom to act as a “leaving group” (such as in diazonium salt methods), leading to poor atom economy. However, this method achieves a dual enhancement of atom economy and step economy through the strategy of“retaining the nitrogen atom and inserting a carbon atom.”
The work of Fan Renhua’s team not only provides an efficient pathway for the synthesis of chiral benzylamines but also opens up new thinking for the functionalization of aromaticC-N bonds. As mentioned in the paper:“This strategy of deconstructing and reconstructing aromaticity is expected to extend to functionalization reactions of other aromatic systems.” In the future, perhaps more“stubborn” aromatic molecules will be successfully modified under this strategy, opening new pathways for drug development and material synthesis.