 High-entropy alloys (HEAs) have become promising candidate materials for hydrogen evolution reaction (HER) electrocatalysts due to their unique cocktail electronic effect and lattice distortion effect.
High-entropy alloys (HEAs) have become promising candidate materials for hydrogen evolution reaction (HER) electrocatalysts due to their unique cocktail electronic effect and lattice distortion effect.Combined experimental and theoretical studies reveal that Pt and Ru serve as the main active sites for HER, while CoNiCu species modulate the electronic density of the active sites to facilitate H* adsorption and achieve optimal M-H binding energy. The hierarchical porous structure and nitrogen doping of the hNCNC support also play a key role in enhancing HER activity and stability.This study presents an effective strategy to significantly enhance noble metal HER performance by developing HEAs on a unique hNCNC support.

01 Catalyst Design and Synthesis
Hierarchical porous nitrogen-doped carbon nanocages (hNCNC) as support material: hNCNC not only provides abundant active sites but also promotes electrolyte wetting and mass transfer through its hierarchical porous structure. 02 Catalyst Characterization
02 Catalyst Characterization
Characterization of electrochemical properties: Electrochemical methods, including cyclic voltammetry (CV) testing and linear sweep voltammetry (LSV) testing, were used to evaluate the catalyst’s HER activity, electrochemical active surface area (ECSA), and charge transfer resistance (Rct).
04 Performance Comparison and Mechanistic Discussion
Comparison of performance with other catalysts: The prepared catalyst was compared with other recently reported HER catalysts, demonstrating its superior HER activity.
Discussion of HER mechanism: Combining theoretical calculations and experimental data, the HER mechanism of the catalyst was discussed, including the formation of active sites, electronic density modulation, and the hydrogen evolution process.
05 Visual Guide
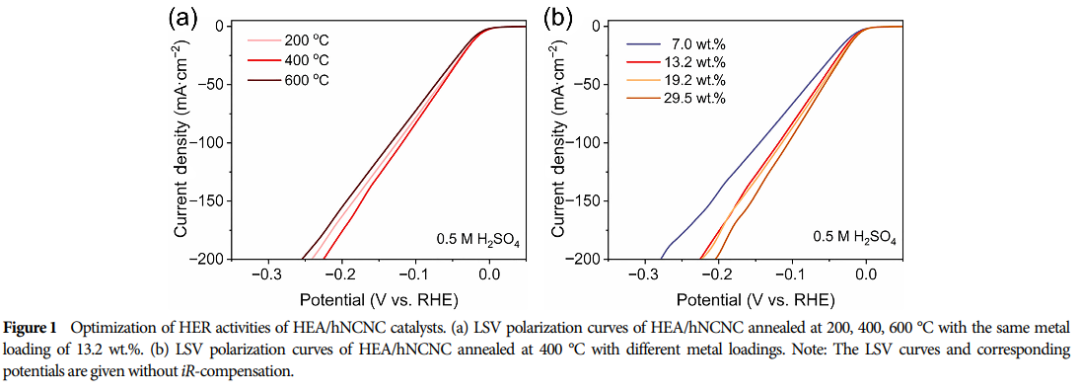
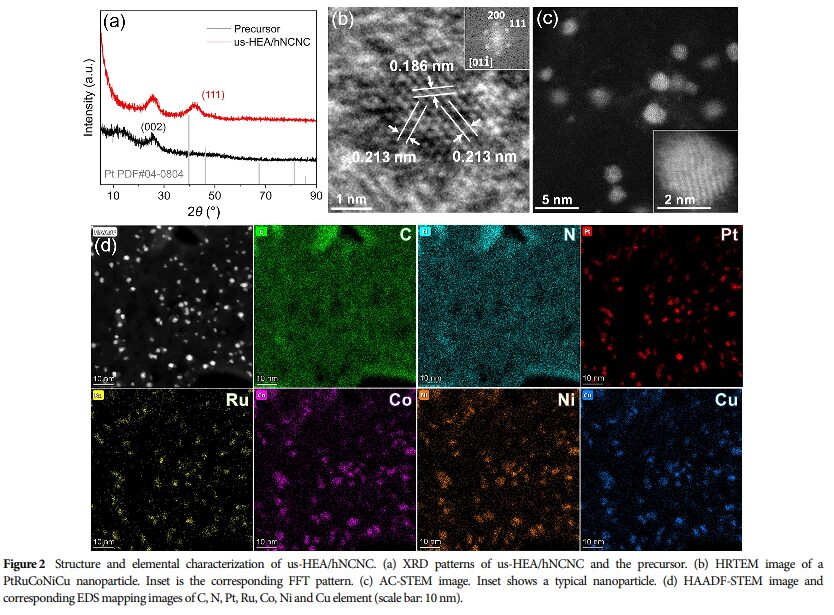
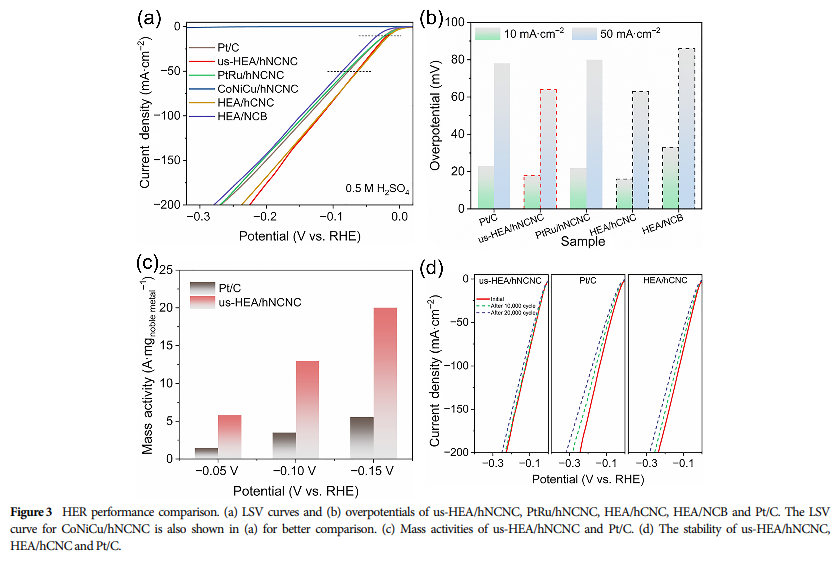

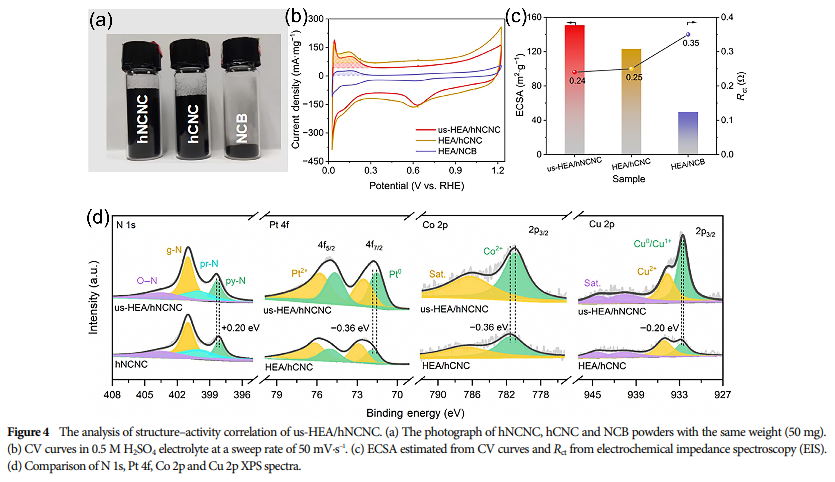
06 Assessment of Catalyst Stability
Long-term stability testing: Long-term HER tests were conducted to evaluate the stability of the catalyst, and the results indicate that the prepared catalyst exhibits excellent stability.
07 Conclusion and Outlook
Research conclusions: This study successfully prepared a high-performance HER catalyst, significantly enhancing its HER activity through the optimization of active sites and pore structure.
Future outlook: Future research will further explore the structure-activity relationship of the catalyst and how to enhance its HER performance through more refined synthesis strategies.
08 Experimental Section
Detailed experimental procedures: The paper describes in detail the synthesis steps of the catalyst, characterization methods, and electrochemical testing conditions, providing a reproducible experimental scheme for other researchers.
-
This paper reveals the preparation methods and performance optimization mechanisms of high-performance HER catalysts through carefully designed experiments and in-depth theoretical analysis, providing strong support for the development of hydrogen energy.
▲ Programmable high-precision membrane electrode coater