
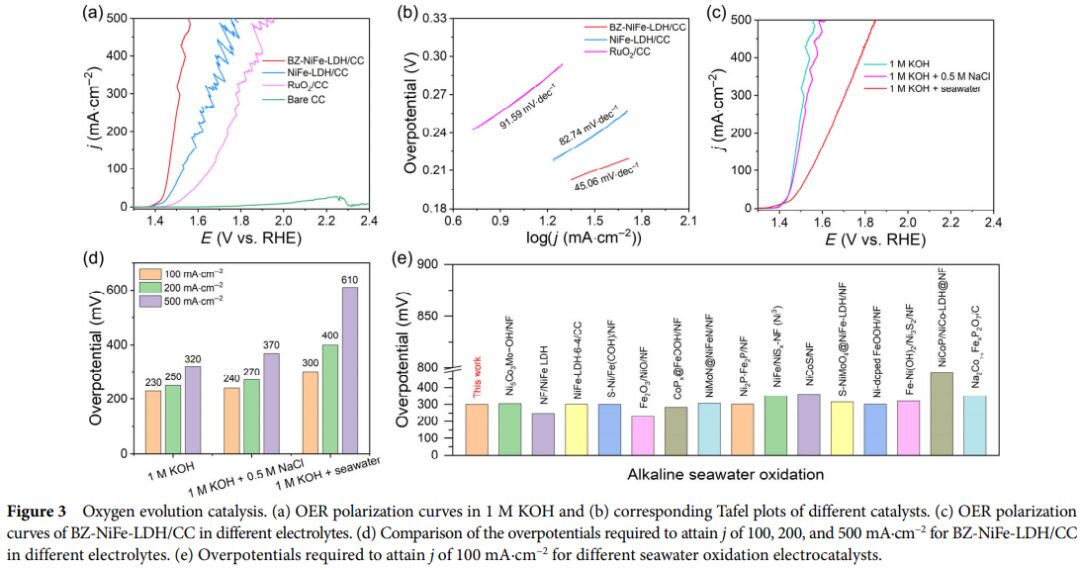
- Seawater electrolysis is an attractive method for clean hydrogen production, but harmful chloride species (i.e., chloride ions and hypochlorite ions) can cause severe corrosion at the anode. Here, we report a recent finding: the carbon cloth-supported NiFe layered double hydroxide nanosheets intercalated with benzoate ions (BZ-NiFe-LDH/CC) serve as an efficient and durable integrated catalyst, showing excellent performance in alkaline seawater oxidation. It increases the interlayer spacing of LDH, suppresses the chlorinated (electro)chemical reactions, and alleviates the local pH drop at the electrode. In a 1 M KOH solution, a current density of 500 mA·cm^-2 can be achieved with an overpotential of only 320 mV. Compared to the rapid activity decay of the NiFe-LDH/CC control sample during long-term electrolysis, BZ-NiFe-LDH/CC stably electrolyzed in alkaline seawater at an industrial-level current density of 500 mA·cm^-2 for 100 hours. In situ Raman spectroscopy studies further reveal the structural changes of the disordered δ(Ni^III-O) during the seawater oxidation process.

- 01 Research Background and Objectives
- This study aims to address the stability and efficiency issues of catalysts during seawater oxidation, with a particular focus on the application of NiFe layered double hydroxides (NiFe-LDH) as catalysts.
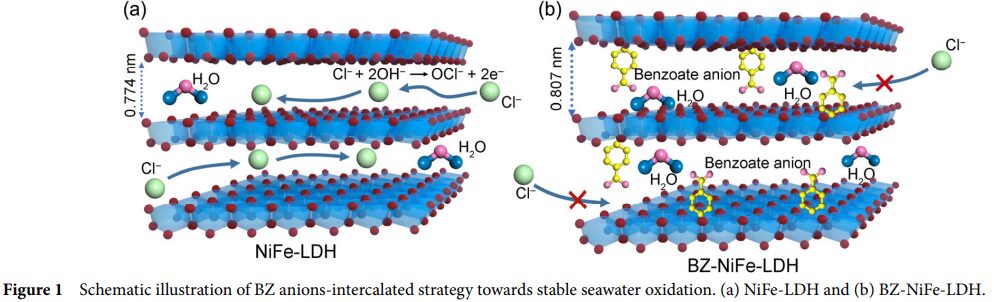
- By intercalating benzoate ions (BZ), the durability and catalytic activity of NiFe-LDH in alkaline seawater oxidation are enhanced.
- 02 Catalyst Design and Preparation
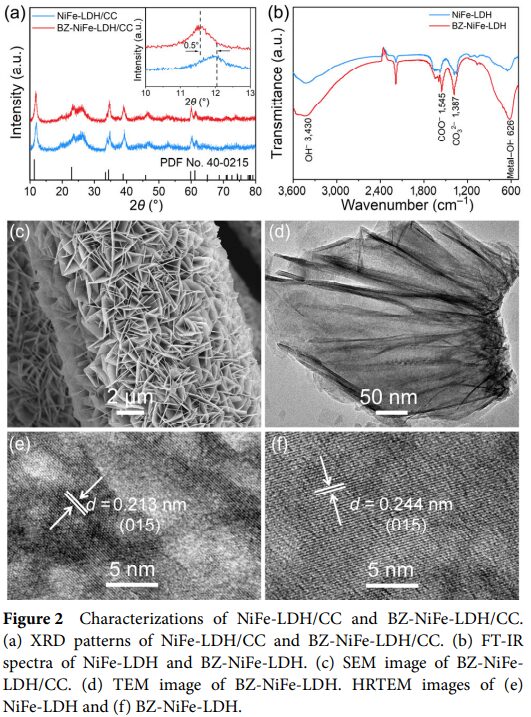
- The research team successfully prepared a nanosheet array of NiFe-LDH intercalated with benzoate ions (BZ-NiFe-LDH/CC) on carbon cloth (CC).
- This structure not only provides a large specific surface area and more active sites but also enhances the stability of the material through the intercalation of benzoate ions.
- 03 Catalytic Performance Evaluation
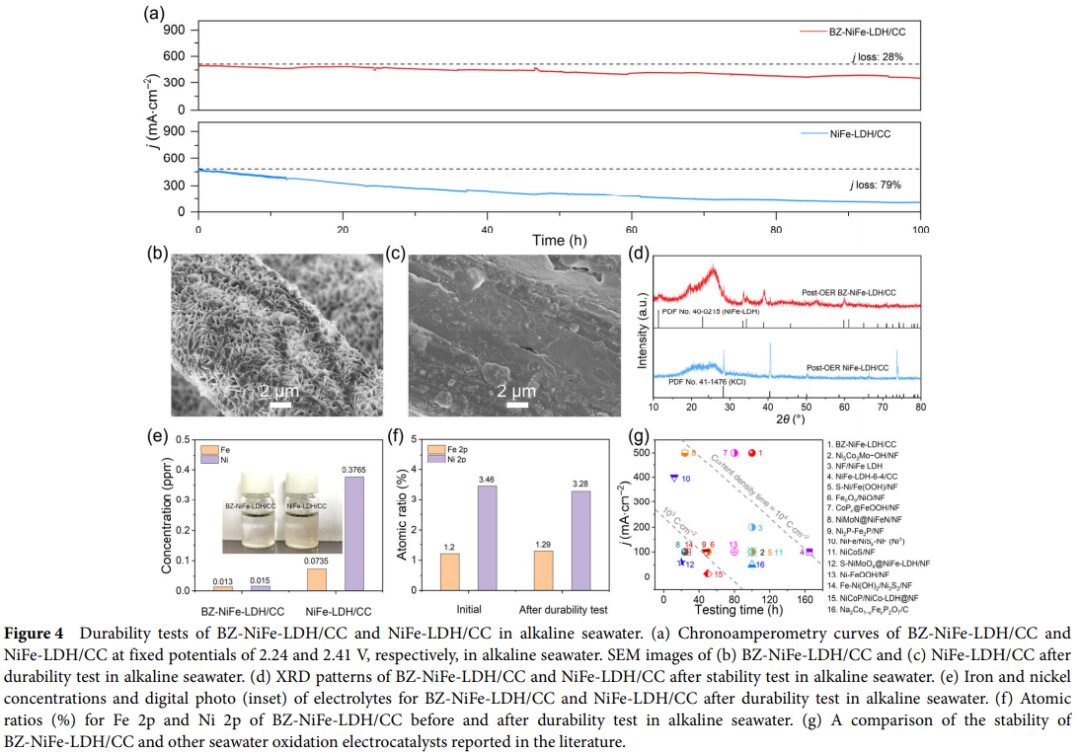
- Through electrochemical performance testing, BZ-NiFe-LDH/CC exhibits excellent catalytic activity in alkaline seawater oxidation, with lower overpotential and significantly better stability compared to non-intercalated NiFe-LDH/CC. Notably, after prolonged electrolysis, BZ-NiFe-LDH/CC still maintains high catalytic activity.
- 04 Structural Characterization and Mechanistic Discussion
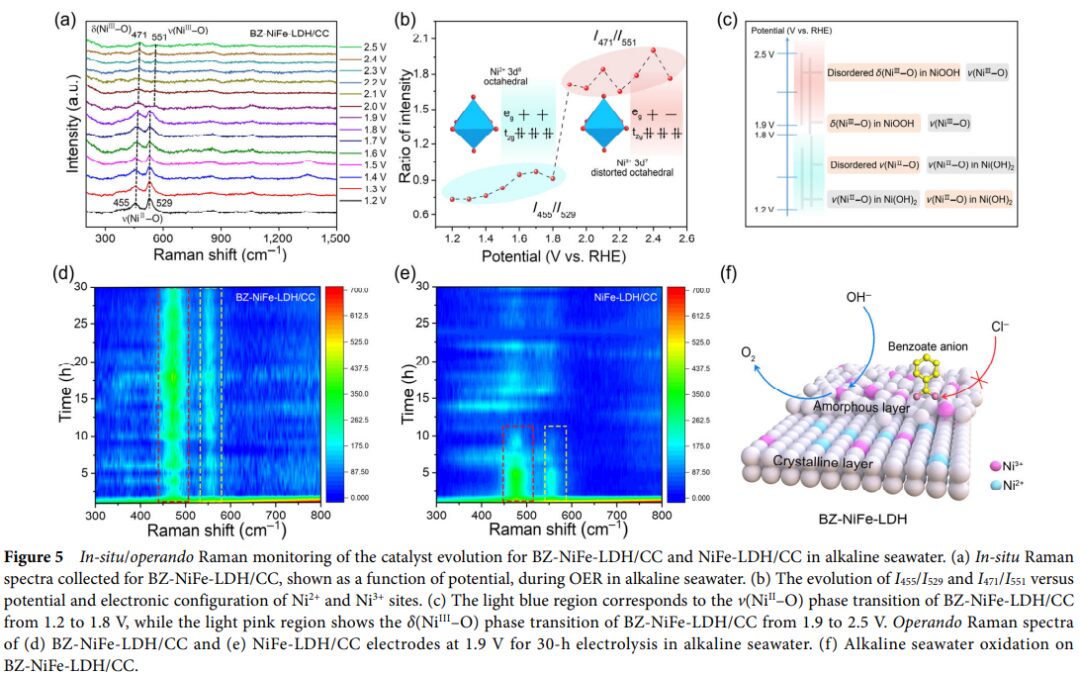
- Using methods such as X-ray diffraction and Raman spectroscopy, the structure of BZ-NiFe-LDH/CC was characterized, revealing that the intercalation of benzoate ions leads to an increase in interlayer spacing, facilitating electrolyte diffusion and exposing active sites. Meanwhile, Raman spectroscopy studies reveal structural changes of the active site δ(Ni III-O) during the seawater oxidation process.

- 05 Role of Benzoate Ions
- Benzoate ions in BZ-NiFe-LDH/CC not only act as proton acceptors, helping to alleviate local pH drops, but also serve as chloride ion repellers, effectively suppressing the harmful electrochemical processes of chloride ions, thereby enhancing the stability and durability of the catalyst.
- 06 Illustrated Guide
- 07 Performance Comparison and Application Prospects
- Comparing the performance of BZ-NiFe-LDH/CC with other self-supporting catalysts, it was found that its performance in alkaline seawater oxidation outperforms many reported catalysts.
- Moreover, this study provides new insights for developing efficient, durable, and cost-effective seawater oxidation catalysts, with broad application prospects.
- 08 Conclusion and Outlook
- The research team summarized the reasons for the excellent performance and enhanced stability of BZ-NiFe-LDH/CC in electrochemical seawater oxidation and looked forward to future research directions in catalyst surface engineering, particularly using negatively charged molecules for surface engineering to improve the durability and efficiency of catalysts.
09 Summary
-
The article successfully prepared the BZ-NiFe-LDH/CC catalyst with high stability and catalytic activity through the benzoate ion intercalation technique, providing a new solution for electrochemical seawater oxidation.
-
This catalyst exhibits excellent performance in alkaline seawater oxidation, with broad application prospects and significant research value.