

01 Research Background
Challenges of Alkaline Hydrogen Evolution: The alkaline hydrogen evolution reaction (HER) faces challenges due to the slow hydrolysis process, especially at high current densities.
Dual Active Site Requirement: Efficient HER electrocatalysts should provide dual active sites to synergistically and separately promote the cleavage of H–O–H bonds and optimal adsorption.
02 Synthesis Method
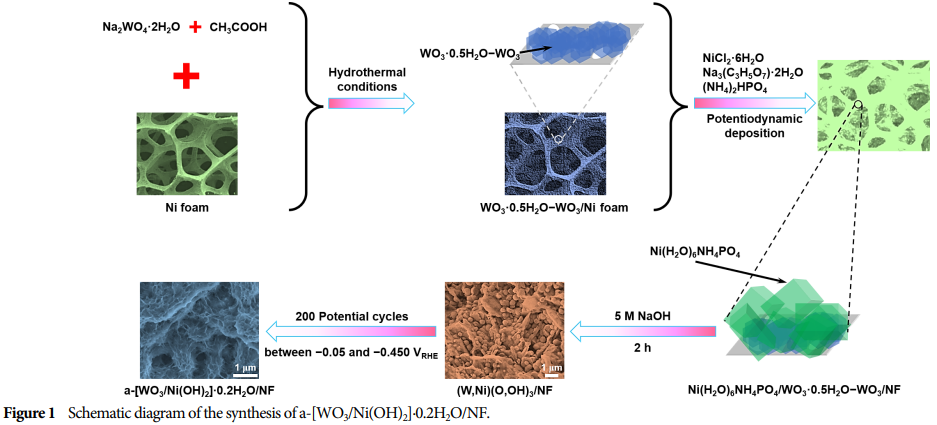
Electrodeposition Method: Amorphous (W,Ni)(O,OH)3 was deposited on Ni(H2O)6NH4PO4/WO3·0.5H2O–WO3/NF substrates using the electrodeposition method.
Subsequent Treatment: Through subsequent treatment, (W,Ni)(O,OH)3 was converted to a-[WO3/Ni(OH)2]·0.2H2O, adjusting the atomic ratio of W and Ni.
03 Electrocatalyst Characterization
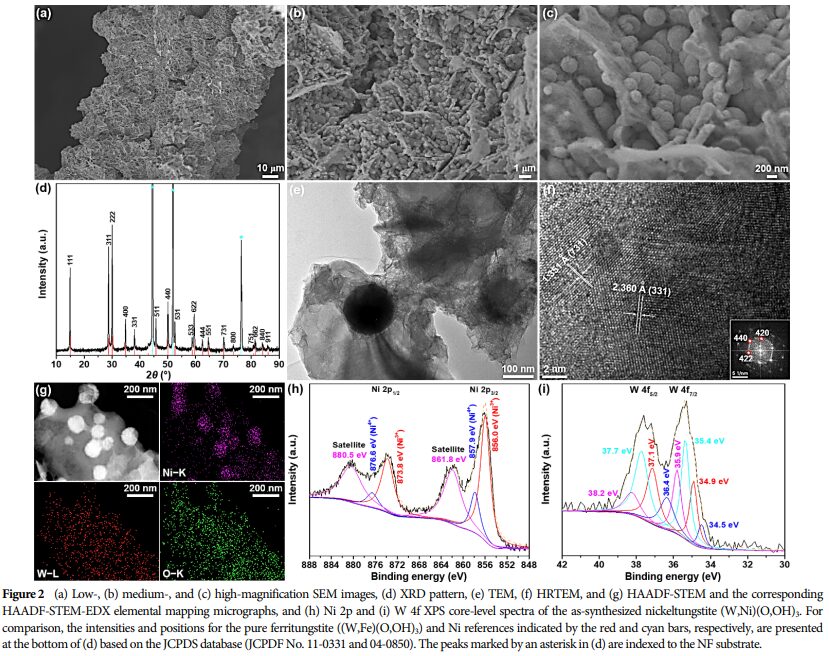
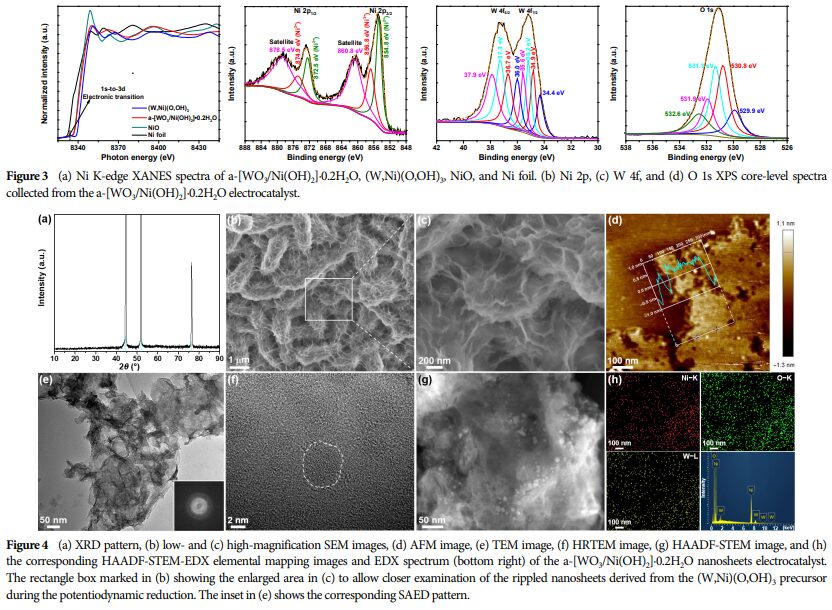
Morphology and Structure: SEM and TEM images showed that the nanosheets have a fish-scale texture and rough surface, indicating distinct amorphous characteristics.
Element Distribution: HAADF-STEM and EDX elemental mapping images indicate that the WO3 component is uniformly embedded in the Ni(OH)2 matrix.
Oxidation State Analysis: Ni K-edge XANES spectra reveal a high oxidation state of Ni species.
04 Electrochemical Performance
Hydrogen Evolution Activity: a-[WO3/Ni(OH)2]·0.2H2O exhibits significant HER activity under alkaline conditions, outperforming a-WO3, Ni(OH)2, and the NF substrate.
Starting Potential: The starting potential of a-[WO3/Ni(OH)2]·0.2H2O is low (-6 mV), better than a-WO3 (-34 mV) and Ni(OH)2 (-37 mV).
High Current Density Performance: At a high current density of -500 mA·cm-2, a-[WO3/Ni(OH)2]·0.2H2O exhibits excellent stability and a small overpotential (-163 mV).
05 Performance Optimization
Effect of W Content: HER activity increases with the increase of W content, but excessive W content can lead to decreased activity.
W/Ni Atomic Ratio: The optimal W/Ni atomic ratio for a-[WO3/Ni(OH)2]·0.2H2O is approximately 1:3.5.

06 Mechanism Analysis
Dual Functional Synergy: The components of WO3 and Ni(OH)2 in a-[WO3/Ni(OH)2]·0.2H2O synergistically promote water dissociation and H2 desorption.
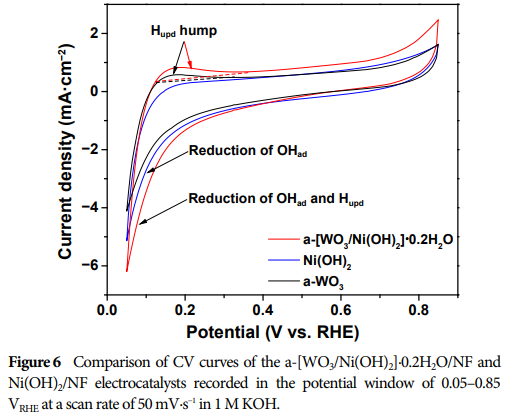
Enhanced Adsorption Capability: The presence of the WO3 component enhances the adsorption capability of H ad and OH ad, thereby improving HER performance.
07 Visual Guide
08 Conclusion and Outlook
Research Conclusion: a-[WO3/Ni(OH)2]·0.2H2O is a cheap, durable, and efficient alkaline HER electrocatalyst with excellent performance and stability.
Future Outlook: By designing finely tuned nanostructures and optimizing WO3 content, it is expected to further enhance the HER performance of a-[WO3/Ni(OH)2]·0.2H2O.
In summary, the article demonstrates the efficient performance of amorphous mixed tungsten oxide-nickel hydroxide nanosheets in alkaline hydrogen evolution reaction through detailed research and experimental data, exploring the mechanisms behind it. These research findings provide important references and insights for developing new, efficient hydrogen evolution reaction electrocatalysts.