Recently, Stony Brook University and the research group led by Michael Mak proposed a new method called “Tunable Rapid Assembly Collagen Elements (TRACE)”, which achieves instant assembly of unmodified collagen through the macromolecular crowding (MMC) effect. This technology represents a significant breakthrough in the fields of tissue engineering and bioprinting, with potential wide applications in regenerative medicine and complex tissue construction. The related paper titled “Instant assembly of collagen for tissue engineering and bioprinting” was published in Nature Materials, with the first authors beingXiangyu Gong, Zhang Wen, and Zixie Liang.

Research Background
Type I collagen is a key scaffold material in human tissues, but its assembly kinetics in vitro are difficult to control, limiting its application as a primary scaffold or cell bio-manufacturing adhesive. Traditional collagen gel preparation methods typically require gelation times of several tens of minutes and struggle to precisely control the assembly process in both spatial and temporal dimensions. Furthermore, existing techniques such as chemical modification or UV crosslinking may affect the physiological relevance and biocompatibility of collagen.
Technical Breakthrough
The research team utilized inert macromolecular crowding agents (such as 8-kDa polyethylene glycol PEG8000) to accelerate the liquid-to-gel transition of collagen, achieving instant assembly of collagen. This method enables efficient construction of physiologically relevant collagen structures at micro to macro scales and promotes cell self-assembly and morphogenesis through the generation of tunable multi-scale structural signals.
The core advantages of TRACE technology include:
1. Rapid Gelation: Collagen begins to gel within 1 second upon contact with PEG solution, completing gelation within 3 minutes, significantly shortening the gelation time required by traditional methods.
2. High Biocompatibility: No chemical modification or UV exposure is required, preserving the natural bioactivity and physiological functions of collagen.
3. Multi-scale Applications: Collagen structures can be precisely constructed from micro-scale collagen fiber bundles to macro-scale tissue structures using TRACE technology.
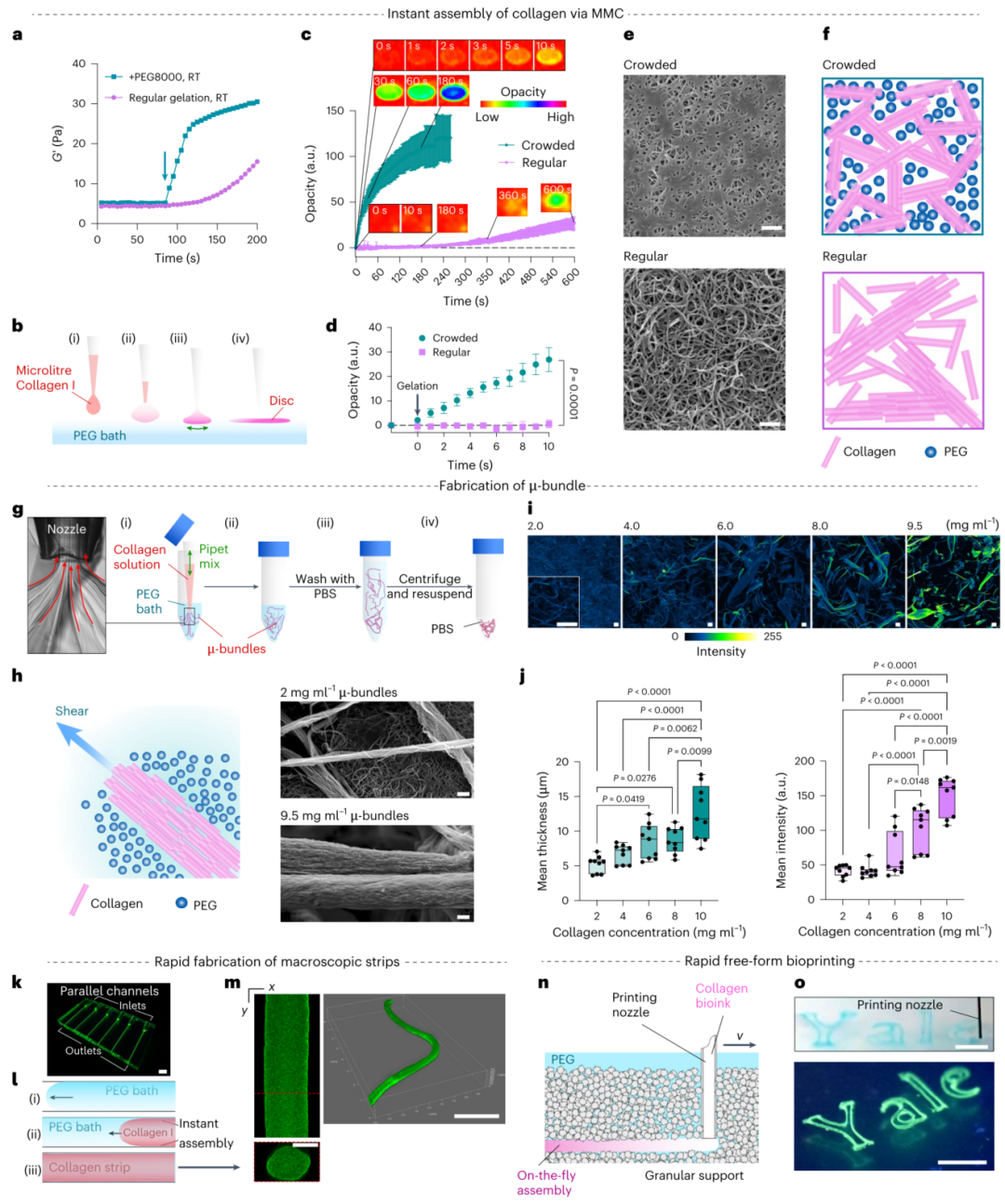
Figure 1. MMC-induced instant gelation facilitates rapid, multi-scale collagen formation.
Application Demonstration
The research team demonstrated the potential of TRACE technology in several fields:
· Vascular Engineering: Guiding endothelial cell self-assembly through collagen micro-bundles (μ-bundles) to form functional networks with angiogenic potential.
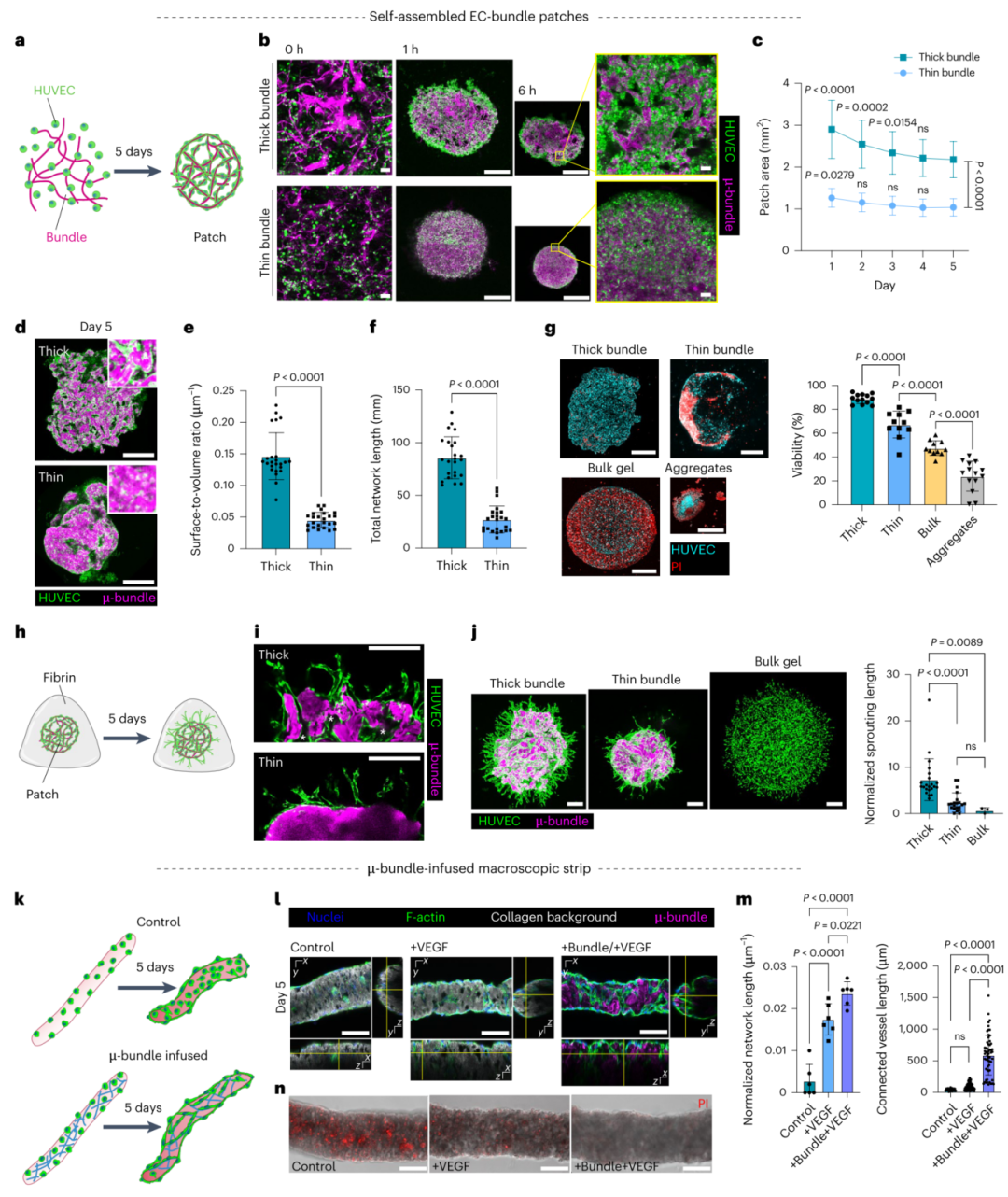
Figure 2. Formation of microvascular systems using collagen bundle scaffolds.
· Intestinal Tissue Construction: Macroscopic strips prepared using the TRACE method successfully differentiated into tubular intestinal tissues with physiological structures.
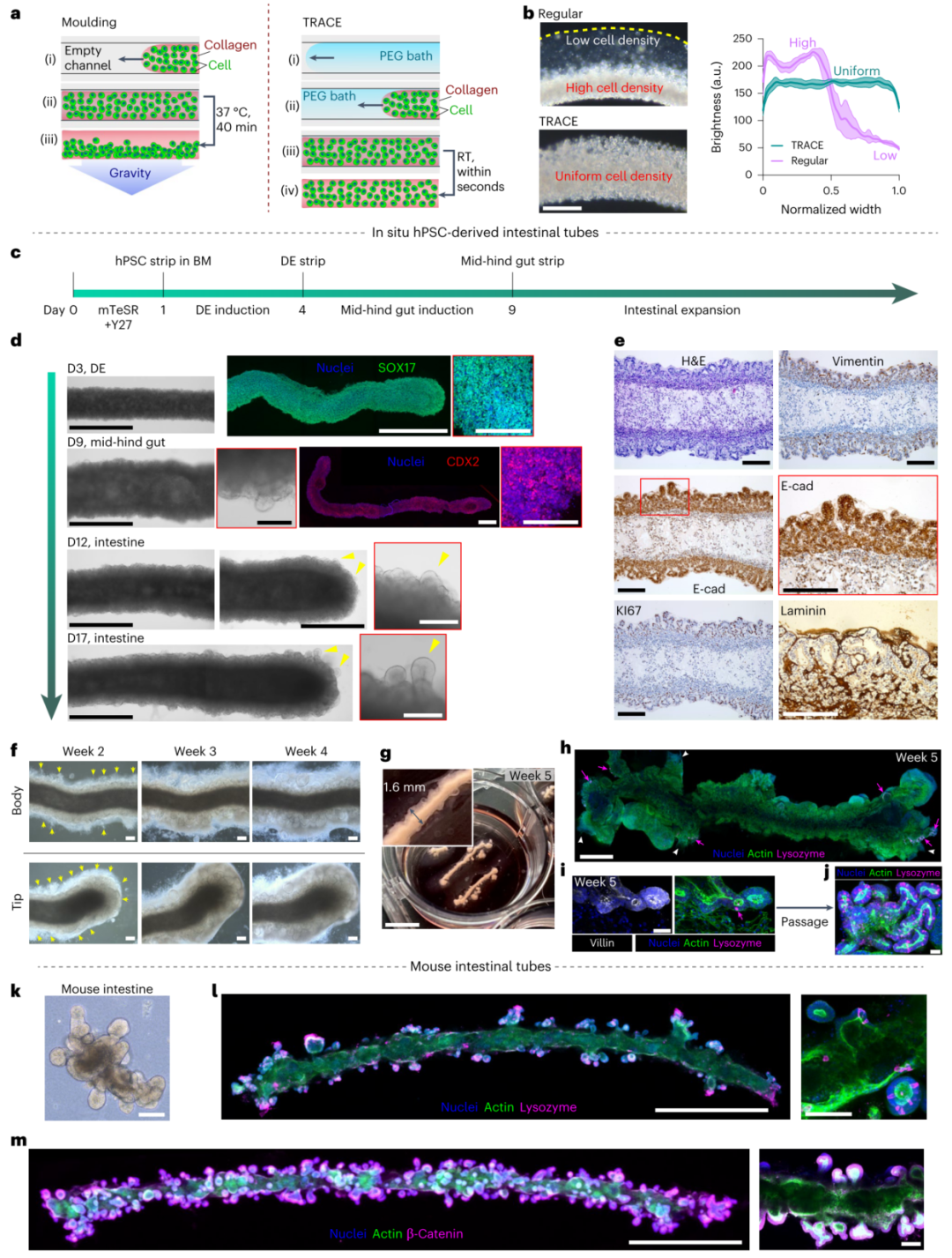
Figure 3. Visible in situ differentiation and self-assembly of intestinal tubes from TRACE strips.
· Bioprinting: TRACE supports precise 3D printing of low-concentration collagen inks, achieving high-fidelity construction of complex structures (such as heart models).

Figure 4. Multi-scale rapid freeform printing of collagen.
· Functional Tissues: Printed cardiac tissues exhibit synchronized calcium transients and fluid pumping functions, simulating the physiological activities of a real heart.
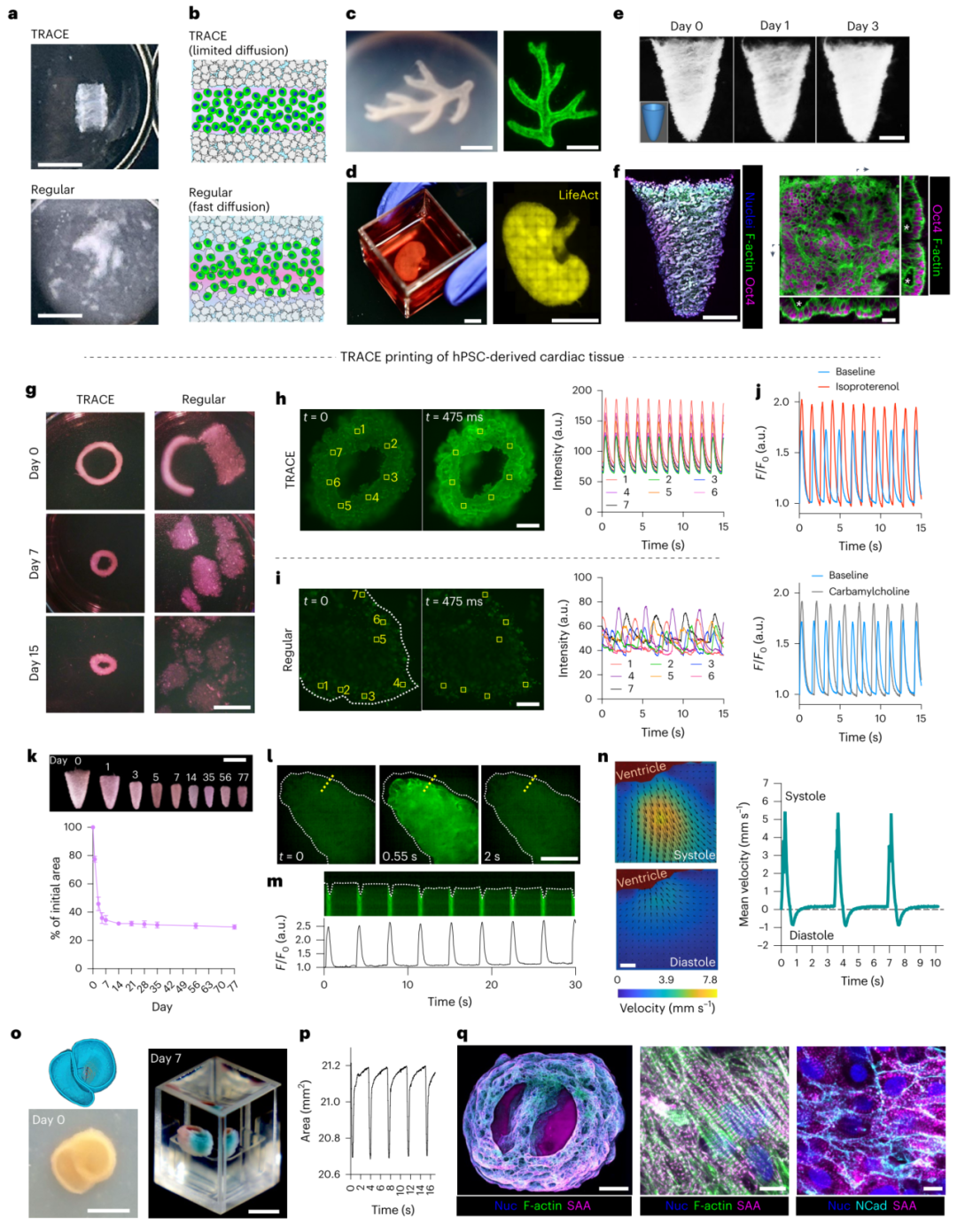
Figure 5. Achieving robust collagen-based cell tissue printing.
Future Prospects
TRACE technology not only expands the application range of unmodified collagen in tissue engineering but also provides new tools for constructing biologically relevant tissues with complex structures and functions. The research team stated that they will further explore the application of the MMC effect in other biomaterials and promote the scalable production of modular tissue units to construct larger, vascularized multifunctional organ systems.
Literature Link
Gong, X., Wen, Z., Liang, Z. et al. Instant assembly of collagen for tissue engineering and bioprinting. Nat. Mater. (2025).
https://doi.org/10.1038/s41563-025-02241-7
Source: Frontiers in Polymer Science
(To ensure the originality of “Today’s New Materials”, “Today’s New Materials” will no longer reprint articles from other public accounts. All articles to be reprinted are stored in the “Yu Qiu” account for reference.)