



Click the blue text above to get more exciting content!

 Abstract
Abstract
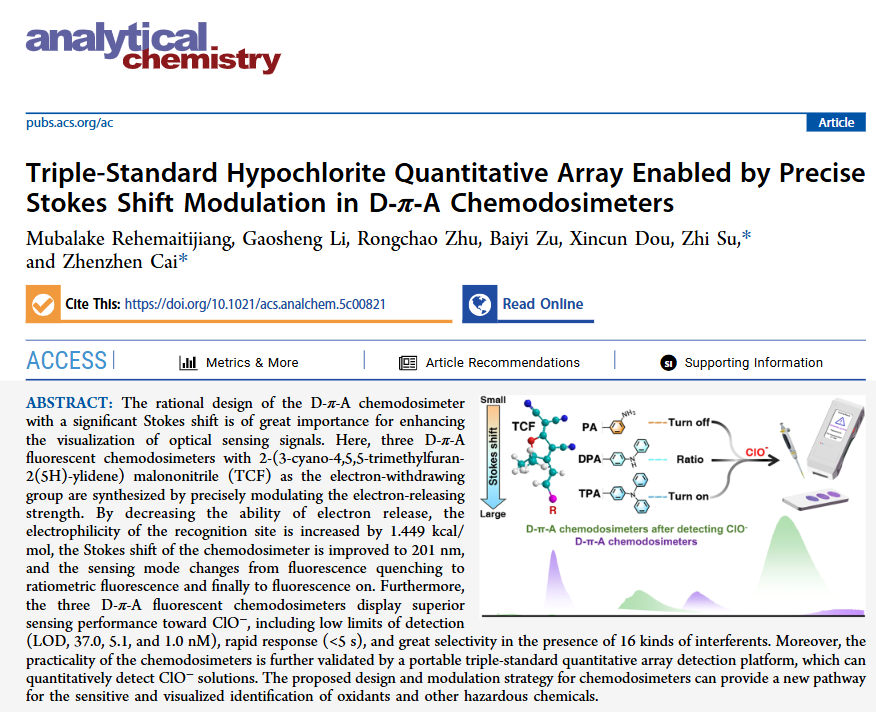
The rational design of D-π-A type chemosensors with significant Stokes shift is crucial for enhancing the visualization of optical sensing signals. Here, three D-π-A type fluorescent chemosensors were synthesized using 2-(3-cyano-4,5,5-trimethylfuran-2(5H)-yl)malononitrile (TCF) as the electron-withdrawing group by precisely adjusting the electron-donating strength. By reducing the electron-donating ability, the electrophilicity of the recognition site was increased by 1.449 kcal/mol, resulting in a Stokes shift of 201 nm for the chemosensor, with the sensing mode transitioning from fluorescence quenching to ratio fluorescence, and finally to fluorescence turn-on. Furthermore, these three D-π-A type fluorescent chemosensors exhibited excellent sensing performance for ClO⁻, including low limits of detection (LOD of 37.0, 5.1, and 1.0 nM, respectively), rapid response (<5 s), and high selectivity in the presence of 16 interfering substances. Additionally, the practicality of the chemosensors was further validated through a portable triple-standard quantitative array detection platform capable of quantifying ClO⁻ solutions. The design and modulation strategies of the proposed chemosensors provide new avenues for the sensitive visualization and identification of oxidants and other harmful chemicals.
 Introduction
Introduction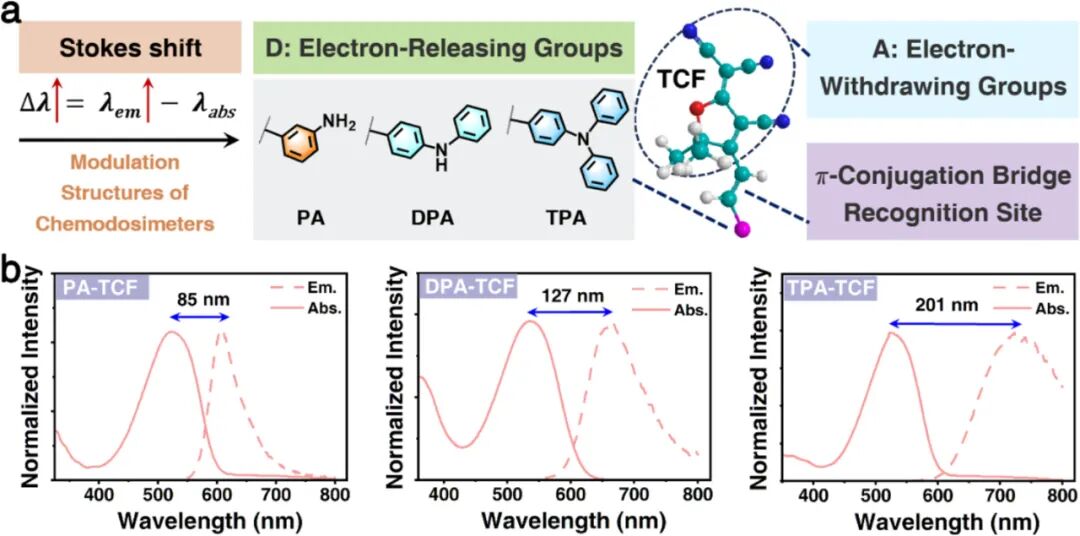
(a) Design strategy of the D-π-A type fluorescent chemosensor based on TCF and a schematic diagram of the precise control of electron-donating strength. (b) Normalized absorption and emission spectra of the TCF-based D-π-A chemosensors PA-TCF, DPA-TCF, and TPA-TCF, with Stokes shifts of 85, 127, and 201 nanometers, respectively (excitation wavelength λex = 395 nanometers, slit: 1.5 nanometers).
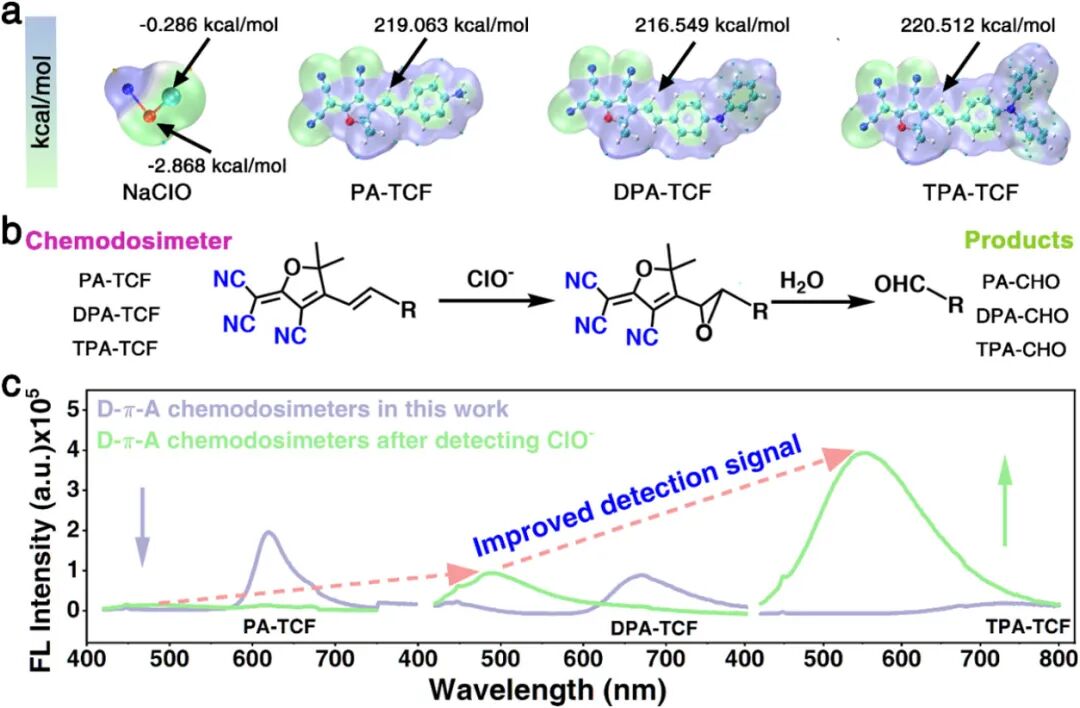
(a) Electrostatic potential surface maps of the D-π-A type chemosensor based on ClO⁻ and TCF. (b) Schematic diagram of the proposed mechanism of the TCF-based D-π-A chemosensor’s interaction with ClO⁻. (c) Fluorescence emission spectra of the TCF-based D-π-A chemosensor before (purple line) and after (green line) detecting ClO⁻ (excitation wavelength λex = 395 nm, slit: 1.5 nm).
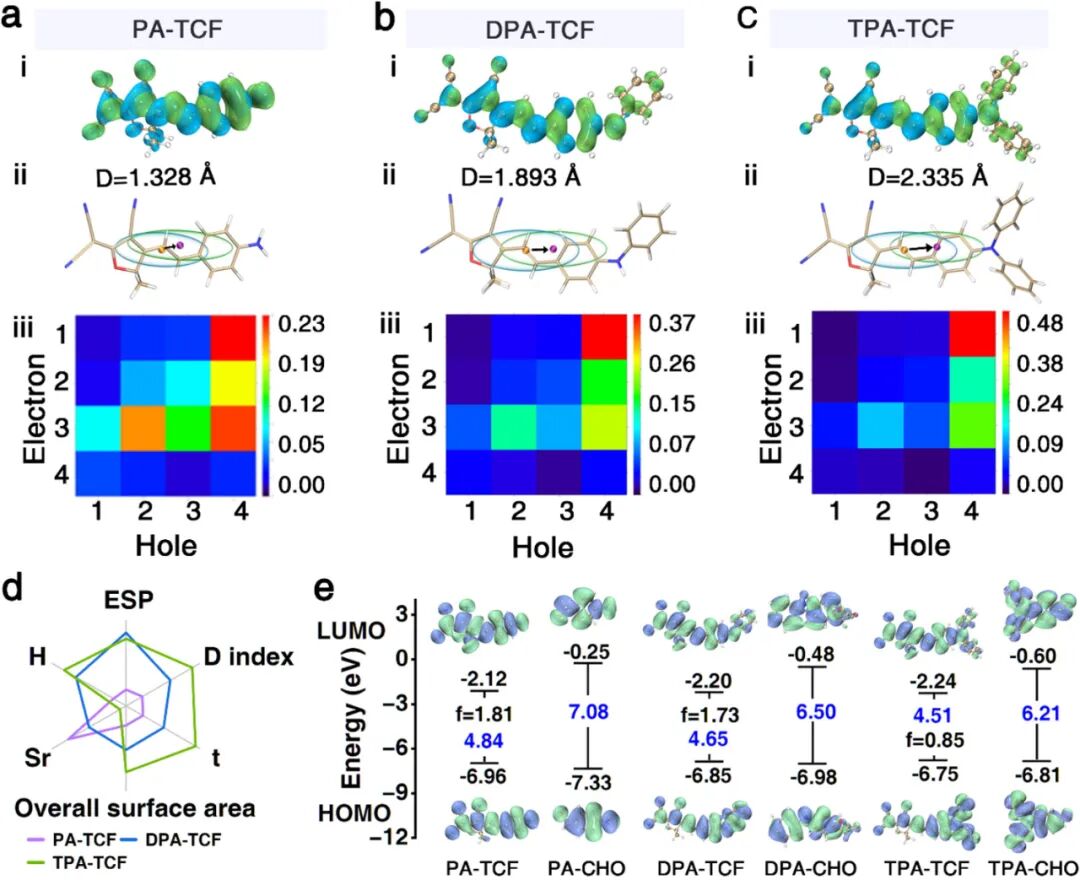
(i) Hole and electron distribution (green and blue areas represent hole and electron distributions, respectively), (ii) Chole/Cele diagram smoothly transitioning from hole and electron distributions, and (iii) fragment transition density matrix diagrams of the chemosensors PA-TCF (a), DPA-TCF (b), and TPA-TCF (c). (d) Radar charts of the main physical quantities of PA-TCF, DPA-TCF, and TPA-TCF. (e) Electron density distributions and corresponding energy level diagrams of the main molecular orbitals of PA-TCF, DPA-TCF, TPA-TCF, and their reaction products PA-CHO, DPA-CHO, and TPA-CHO. The purple and green areas represent positive and negative orbital phases, respectively.
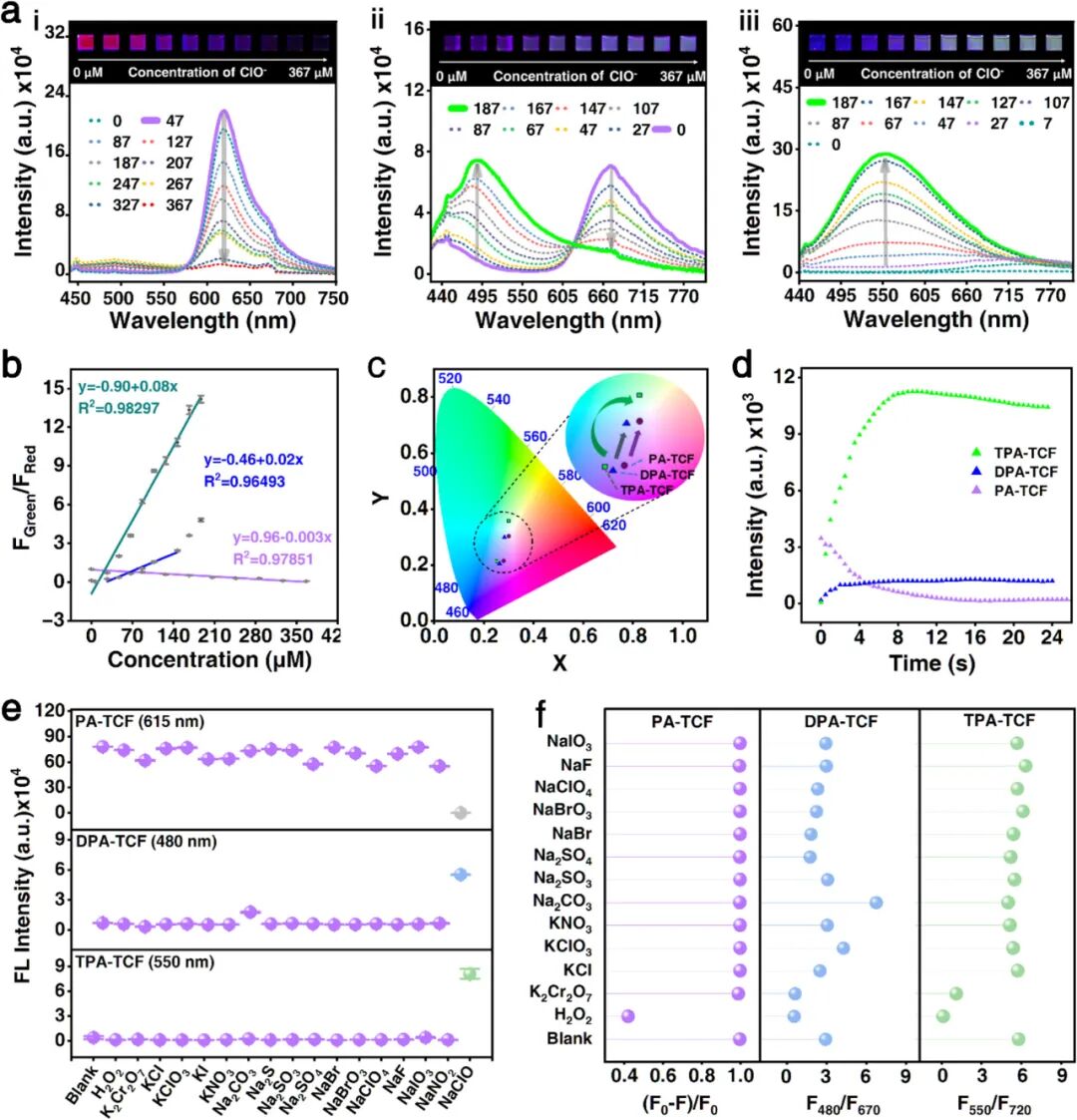
(a) Fluorescence spectra of PA-TCF (i), DPA-TCF (ii), and TPA-TCF (iii) (10 micromolar) under different concentrations of ClO⁻ (0 – 367 micromolar) in tetrahydrofuran, along with corresponding fluorescence images under 395 nanometer UV light. (b) Emission intensity ratios (F500 / F620, F480 / F670, F550 / F720) of chemosensors PA-TCF (purple line), DPA-TCF (blue line), and TPA-TCF (green line) as functions of ClO⁻ concentration. (c) Evaluation of fluorescence color changes of PA-TCF, DPA-TCF, and TPA-TCF after adding ClO⁻ based on the CIE 1931 chromaticity diagram. (d) Time-resolved fluorescence responses of PA-TCF, DPA-TCF, and TPA-TCF after adding ClO⁻. (e) Fluorescence intensity responses of PA-TCF, DPA-TCF, and TPA-TCF (10 micromolar) to various interfering substances (50 micromolar) at 620 nanometers, 480 nanometers, and 550 nanometers. (f) Quenching efficiency of PA-TCF ((F0 – F)/F0) and fluorescence intensity ratios of DPA-TCF and TPA-TCF (10 micromolar) under coexistence of various interfering substances (50 micromolar) and ClO⁻ (1 millimolar).
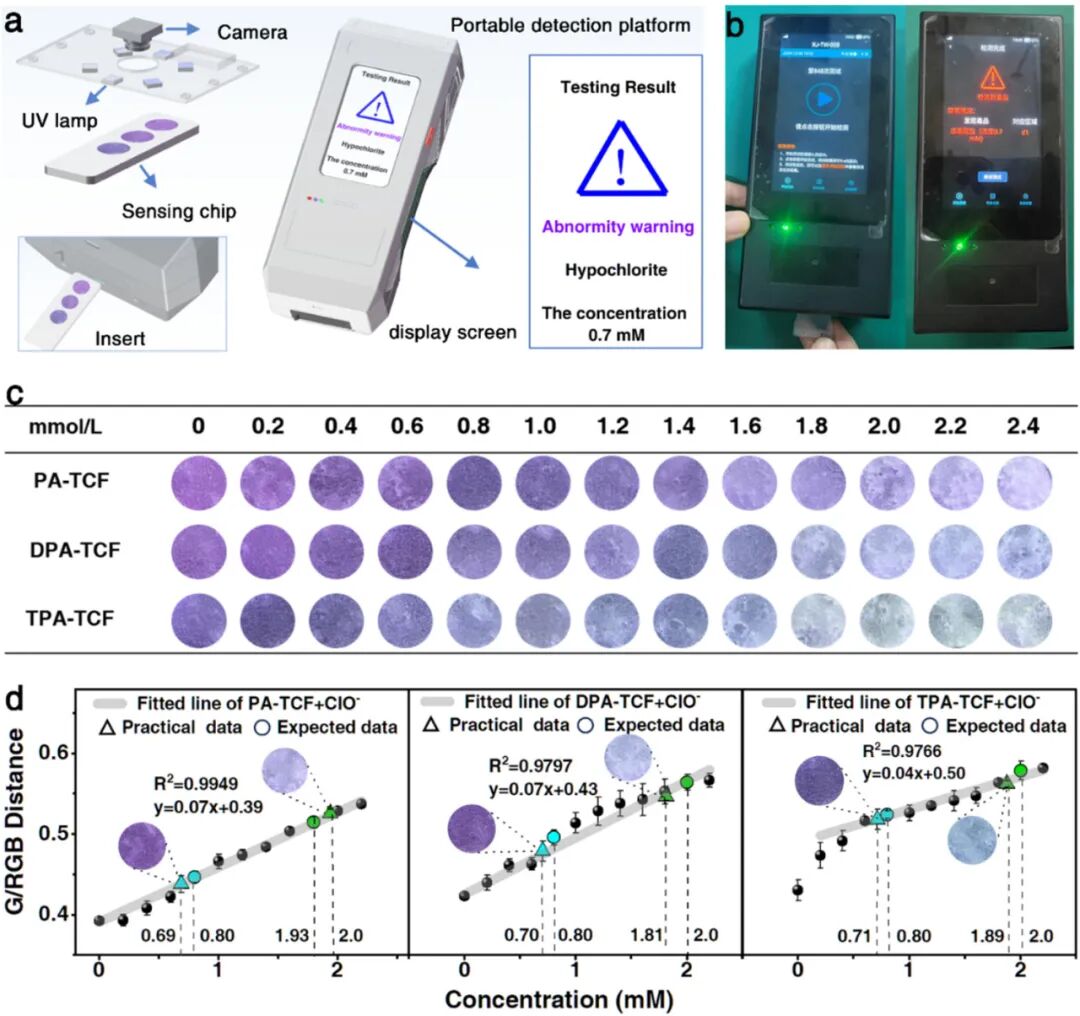
(a) Schematic diagram of the working principle of the portable triple-standard quantitative array detection platform. (b) Image of the porous polymer-based device inserted into the portable triple-standard quantitative array detection platform and alarm display after detecting ClO⁻. (c) Fluorescence images of the porous polymer sensor unit loaded with three chemosensors under different concentrations of ClO⁻ solutions (under 395 nanometer UV light). (d) Correspondence between the G/(RGB distance) values extracted from images of different concentrations of ClO⁻ solutions and the two actual application test results marked on the standard line (marked as cyan and green, respectively).
Related results were published in the international academic journal Analytical Chemistry as “Triple-Standard Hypochlorite Quantitative Array Enabled by Precise Stokes Shift Modulation in D-π-A Chemodosimeters”.
Literature link: Click to read the original text
https://doi.org/10.1021/acs.analchem.5c00821
Disclaimer:The original content only represents the original translation, with limited proficiency, and is for academic exchange only. This platform does not claim copyright of the original text. If there is any infringement, please contact us for deletion. We apologize for any omissions in the literature interpretation. Please contact the author team promptly (WeChat: analytichemistry, email: [email protected]), and we will make corrections or reissue the manuscript as soon as possible. Thank you for your understanding!


For submissions, recommendations, and collaborations, please contact us at: [email protected]
 For WeChat communication and collaboration, please add the editor’s WeChat.
For WeChat communication and collaboration, please add the editor’s WeChat.