
Not all long-established ADC Biotechs can achieve a successful exit like Seagen (Seattle Genetics).
Today, Mersana Therapeutics, a 23-year-old veteran biotech, has reached its most desperate moment. With a cumulative deficit of $900 million and only $134.6 million left in cash, its two core pipeline products are still in Phase I clinical trials.
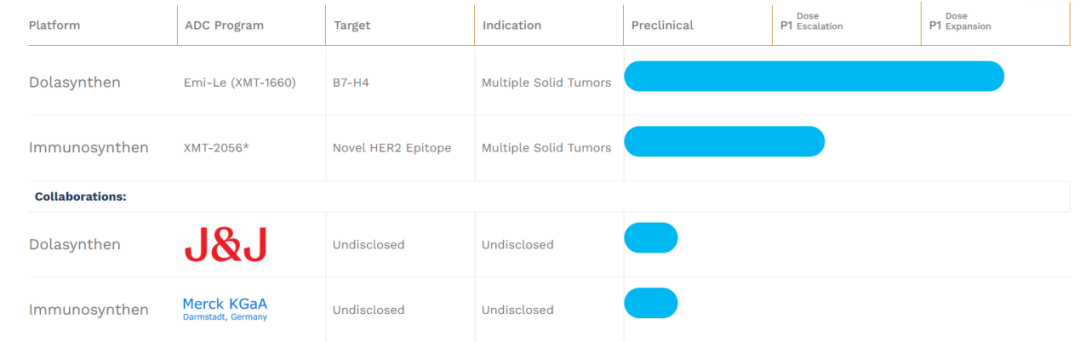
To sustain operations until the third quarter of next year, the company has decided to lay off an additional 55% of its workforce on top of the previously announced 50% reduction in 2023. The number of employees may drop below 50 due to this layoff. The current focus of the core pipeline product XMT-1660 will be set on breast cancer. They also plan to continue the Phase I dose escalation work for XMT-2056 and maintain ongoing collaborations with Johnson & Johnson and Merck.
How did it come to this?
From a technical perspective, Mersana Therapeutics has innovative technology platforms, such as DolaLock, Dolaflexin, and Immunosynthen.
1. DolaLock aims to address the bystander effect: traditional ADC drugs may cause the cytotoxic drug to diffuse to surrounding healthy cells after release, resulting in unnecessary toxicity due to the bystander effect.
In contrast, ADCs designed with DolaLock technology prevent the metabolic products of the cytotoxic drug from crossing the cell membrane after release, effectively limiting the range of the bystander effect.
2. Dolaflexin utilizes a biodegradable polymer called Fleximer, capable of carrying multiple drug molecules, significantly improving drug solubility, pharmacokinetics, and immunogenicity, and greatly increasing the number of drug molecules carried by each ADC (DAR approximately 10), thereby enhancing efficacy.
3. The Immunosynthen platform employs STING agonists conjugated to antibodies to locally activate STING signaling in tumor-resident immune cells and antigen-expressing cells, unleashing the innate immune potential against tumors.
However, these technologies seem to have had the opposite effect in practice. This may be due to the complexity of the technology itself and the low stability of the actual ADCs.
Mersana’s early ADC product HER2-ADC XMT-1522 was halted in clinical research by the FDA due to a drug-related patient death, ultimately leading to its development termination.
Another early product, XMT-1592, achieved irreversible site-specific conjugation using GlycoConnect™ technology and introduced DolaLock’s AF-HPA payload via Dolasynthen technology, but disappeared for unknown reasons.
More critically, the core pipeline, NaPi2b ADC Upifitamab Rilsodotin (UpRi), which incorporates Dolaflexin technology, reported five fatal bleeding events just before the clinical trial results were to be announced in 2023, leading the FDA to suspend two studies (UP-NEXT/UPGRADE-A).
Moreover, the clinical outcomes have been disappointing, with the pivotal Phase III trial UPLIFT failing to meet its primary endpoint, showing an objective response rate (ORR) of only 13.1% in treated patients, and even among NaPi2b positive patients, the ORR was only 15.6% (vs. over 12% in the control group).
These two failures directly resulted in the company laying off 50% of its workforce.
Concerns About Existing Pipelines
Currently, Mersana’s only remaining pipelines are XMT-1660 and XMT-2056; however, XMT-2056 experienced a Grade 5 (fatal) serious adverse event (SAE) in the first dose group during dose escalation in March 2023. This technology utilizes the Immunosynthen platform.
Although XMT-2056 was cleared for clinical trials in November of the same year, it is strange that by 2025, XMT-2056 is still undergoing dose escalation… A similar situation is observed with XMT-1660; both are in Phase I clinical trials that began in 2022, yet the company seems to be exploring the maximum tolerated dose without any results to date.
Looking directly at the data from 2025, the expanded starting dose selected in the Emi-Le (XMT-1660) drug trial is 67.4 mg/m². If converted based on an average adult body surface area of 70 kg, this dose is approximately equivalent to 1.67 mg/kg, which is evidently low. Moreover, this expanded starting dose is actually the upper limit of the intermediate dose.
Perhaps Mersana’s prolonged exploration of dosage is due to a genuinely narrow therapeutic window.
References:
