Expert Forum

Authors: Xue Jiaohao1, Li Zhipeng1, Zhao Yuanli2
Affiliations: 1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, 2Department of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences
Corresponding Author: Zhao Yuanli
Funding Project: Central High-Level Hospital Clinical Research Special Project (2023-PUMCH-E-011)
Source: Chinese Journal of Medicine, 2025, 16(2): 277-284.
Cerebrovascular diseases are a class of diseases characterized by ischemic or hemorrhagic damage to brain tissue due to vascular lesions, with high incidence, disability, and mortality rates[1-2]. Globally, cerebrovascular diseases have become one of the leading causes of death and long-term disability, imposing a heavy economic and psychological burden on patients and their families, while also posing a significant challenge to social medical resources[3]. In China, the incidence and prevalence of cerebrovascular diseases have been rising annually, making prevention and treatment a pressing public health issue.
Organoid technology has rapidly developed in recent years as a significant breakthrough in the biomedical field. Organoids are miniature organ models with certain tissue structures and functions, formed by culturing stem cells or tissue cells in vitro, capable of simulating the development, physiology, and pathology of in vivo organs[4]. Since the successful cultivation of intestinal organoids by Hans Clevers’ team in 2009, significant progress has been made in organoid technology across multiple organ systems[5]. In the field of cerebrovascular diseases, organoid technology shows great application prospects. By constructing brain organoids, researchers can simulate the pathogenesis of cerebrovascular diseases in vitro, deeply explore key aspects such as angiogenesis and neurovascular unit interactions, and provide a new research platform for early diagnosis, discovery of therapeutic targets, and drug development[6-8]. Despite the significant potential of organoid technology in cerebrovascular disease research, it still faces many challenges, with vascularization being a key bottleneck restricting its further development. Vascularization is crucial for the growth, maturation, and functional maintenance of organoids; a lack of a complete vascular system leads to insufficient nutrient and oxygen supply within the organoids, accumulation of metabolic waste, and affects their long-term survival and functionality[9].
Given the potential and challenges of organoid technology in the research and treatment of cerebrovascular diseases, this article systematically elaborates on the application progress of organoid technology in this field, deeply discusses the construction methods of vascularized brain organoid models, analyzes the research results of drug studies and disease mechanism studies based on these models, and evaluates their application prospects in the treatment of cerebrovascular diseases.

1
Construction of Vascularized Brain Organoid Models
1.1
In Vivo Transplantation
One of the primary methods for generating vascularized brain organoids is to implant them into animal models[10-12]. A study used a lentiviral vector to label green fluorescent protein (GFP) in human embryonic stem cells (hESCs) to cultivate brain organoid embryoid bodies (EBs), which were carefully cultured in vitro for 40-50 days to ensure that these organoids had clear embryoid boundaries, radially arranged neuroepithelium, and well-defined buds[10].
After strict screening, these organoids were transplanted into the posterior cortex of adult immunodeficient mice (NOD-SCID). The transplantation surgery involved creating a cavity in the mouse brain, implanting the organoids, and sealing them with a cover slip and adhesive to form a cranial window. Figure 1 shows a schematic diagram of the construction of the organoid model implanted in the mouse body.
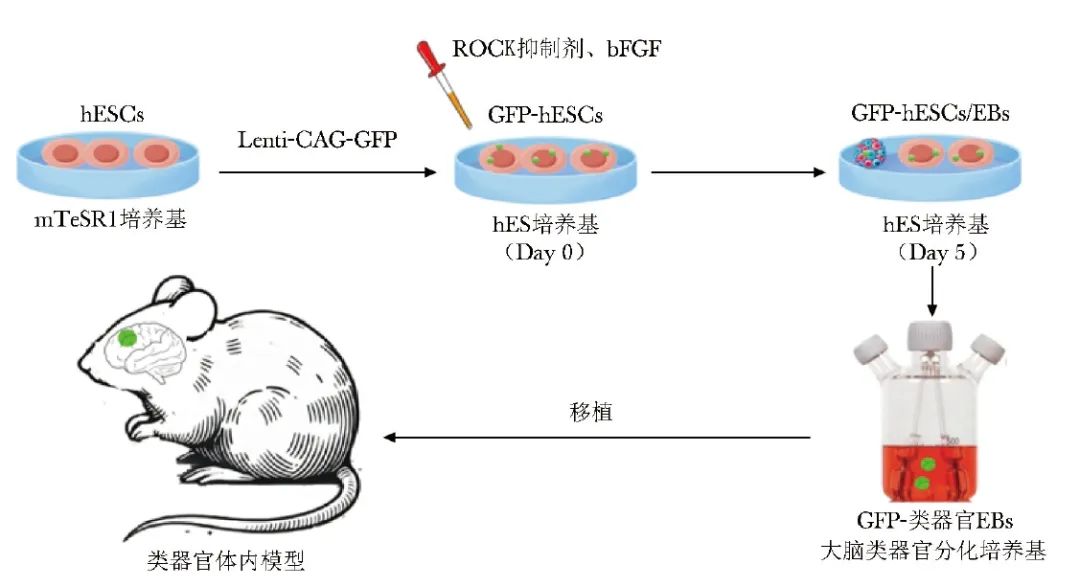
Figure 1 Schematic diagram of the in vivo transplantation organoid model
hESCs (human embryonic stem cells): human embryonic stem cells; GFP (green fluorescent protein): green fluorescent protein; ROCK (Rho-associated protein kinase): Rho-associated protein kinase; bFGF (basic fibroblast growth factor): basic fibroblast growth factor; EBs (embryoid bodies): embryoid bodies
7-10 days post-surgery, host blood vessels began to invade the organoids, and by 14 days post-surgery, a widespread vascular network was formed, with a vascularization success rate of (85.4±6.4)%, and all these vessels originated from the host. This process not only significantly improved the survival rate of the organoids but also promoted the progressive differentiation and maturation of neurons, the generation of glial cells, the integration of microglia, and the extension of axons into multiple regions of the host brain. Using in vivo two-photon imaging technology, researchers directly observed the presence of functional neuronal networks and blood vessels within the grafts. Further in vivo extracellular recordings combined with optogenetics revealed the activity of neurons within the grafts and confirmed the establishment of functional synaptic connections between the grafts and the host brain.
The above findings indicate that transplanting brain organoids into a vascular-rich microenvironment can effectively utilize the natural angiogenic capacity of the host to achieve vascularization and functionalization of the organoids. Overall, the in vivo transplantation strategy for brain organoids successfully overcomes the limitations of necrotic cores by providing effective perfusion, significantly enhancing the vitality of the organoids.
The in vivo transplantation organoid model compensates to some extent for the lack of interaction between the vascular system and the complete physiological environment of the human brain in the in vitro organoid models, but this technology still faces many issues. Firstly, the vascular components in the model are derived from rodents, which limits its translatability to humans and hinders the clinical translation of the model. Secondly, the observed in vivo transplanted organoid models resemble late embryonic or early postnatal tissues, and their maturity and spatial structure still differ from normal vasculature. Additionally, how to accurately monitor and quantify the functional performance of the model, especially the complex changes in blood flow, vascular wall stress, and deformation, remains a significant technical challenge.
1.2
In Vitro Culture
1.2.1 Endothelialization of Organoids
A key challenge in generating vascularized brain organoids is coordinating the inductive factors for different germ layers and cell fates, which often exhibit mutual inhibition. Therefore, some research protocols choose to culture brain organoids and vascular organoids separately. Other methods involve directly introducing non-ectoderm-derived cells or their progenitors into brain organoids to form multi-lineage assemblies[13-15].
A pioneering study attempted to generate homologous structures derived from the same patient[12]. This study utilized patient-derived human induced pluripotent stem cells (hiPSCs) to separately generate brain organoids and endothelial cells (ECs), which were then co-cultured after differentiation. The study activated mesodermal cells through Wnt signaling and differentiated them into endothelial progenitor cells using bone morphogenetic protein 4 (BMP4), vascular endothelial growth factor (VEGF), and fibroblast growth factor 2 (FGF2). On day 34 of differentiation, the brain organoids were re-embedded in a Matrigel matrix containing endothelial progenitor cells and continued to be cultured in vitro for 3-5 weeks, ultimately forming organoids with robust vascular structures. Although the study did not provide a detailed description of the cellular structure in the neuronal compartment, multiple penetrating and perfusable blood vessels appeared in the organoids. These vessels expressed human EC markers such as cluster of differentiation 31 (CD31), proving that patient-derived ECs could be successfully used for the vascularization of brain organoids.
Subsequently, related studies co-cultured hESCs or hiPSCs with human umbilical vein endothelial cells (HUVECs), successfully constructing brain organoid models with vascular structures (vOrganoids)[16]. During the culture process, cells first aggregated into embryoid body-like structures, followed by neural induction and differentiation. Researchers found that HUVECs formed reticular and tubular structures in vOrganoids, which were mainly distributed above the ventricular/subventricular zone, an area rich in neural stem cells and progenitor cells. As development progressed, the vascular structures continuously extended towards the newly formed neuronal regions, and this state could be maintained for over 200 days. Electrophysiological tests further revealed the presence of spontaneous excitatory postsynaptic currents, spontaneous inhibitory postsynaptic currents, and bidirectional electrical transmission in vOrganoids, indicating that vOrganoids possess chemical and electrical synapses. Through single-cell RNA sequencing analysis, researchers confirmed that vOrganoids exhibit high similarity in cell types and molecular properties to human fetal forebrain and found that the developmental speed of vOrganoids is faster than that of non-vascularized organoids. Additionally, in vivo transplantation experiments confirmed that vOrganoids could integrate well with host brain tissue and establish functional connections with the host vascular system.
Another vascularization strategy for brain organoids involves genetic modification to induce EC differentiation during the formation of brain organoids[14]. Studies have shown that generating human cortical organoids from hESCs and genetically modifying them can ectopically express the human ETS variant 2 (ETV2). ETV2 can promote the differentiation of hESCs into ECs, a process that does not rely on specific differentiation conditions or growth factors (such as VEGF). The optimal ETV2 expression cell ratio (20%) was determined, and ETV2 expression was induced starting on day 18, resulting in organoids that effectively formed vascular-like structures. These structures not only exhibited morphological characteristics of EC marker expression but also functionally supported the transport of oxygen and nutrients, reduced cell death, and promoted neuronal maturation. Furthermore, these vascularized organoids also exhibited blood-brain barrier (BBB) characteristics, including tight junctions, increased expression of nutrient transport proteins, and increased transendothelial electrical resistance. Single-cell transcriptome analysis further confirmed that vascularization promotes neuronal maturation. An important feature of this model is that the vascular structures are surrounded by astrocytes and pericytes, closely resembling the composition of the human neurovascular unit. This method, mediated by a single transcription factor, can induce vascularization across germ layers and achieve precise control over the timing of cell fate induction within organoids.
1.2.2 Fusion of Vascular Organoids
As an innovative alternative, a study proposed a new strategy of mixed neurovascular spheroids to achieve effective vascularization of brain organoids[17]. Researchers independently cultured induced neural progenitor cells (iNPCs) and induced endothelial cells (iECs) spheroids based on hiPSCs; subsequently, human mesenchymal stem cells (MSCs) and reagents such as SB431542 and LDN193189 were added to the mixed culture medium to form iNPC-iEC-MSC fused spheroids. Figure 2 shows a schematic diagram of the construction of the in vitro spheroid fusion organoid model.
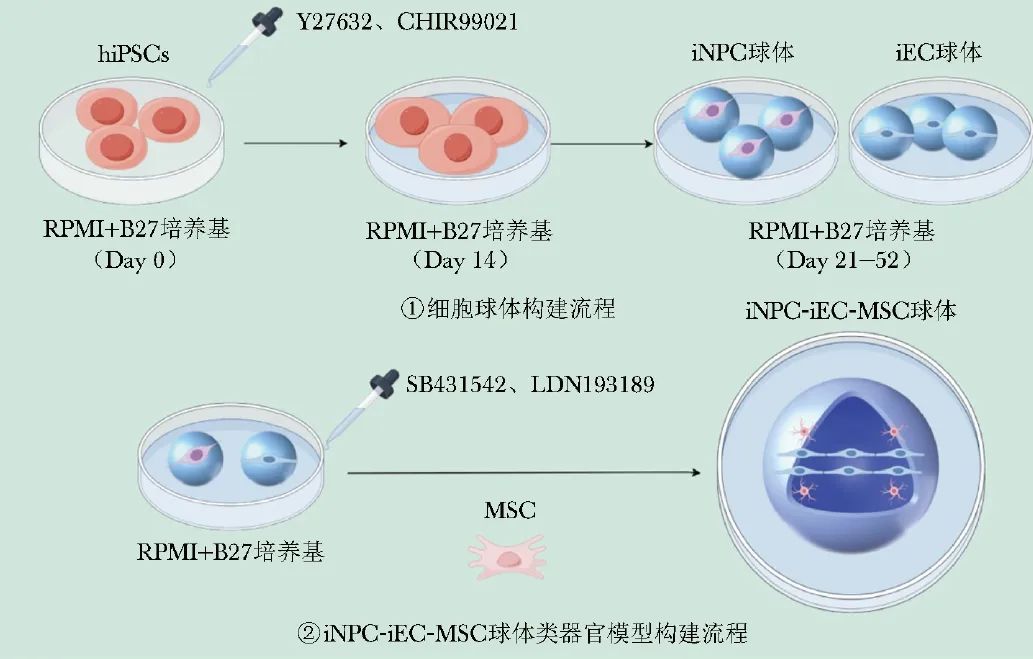
Figure 2 Schematic diagram of the in vitro spheroid fusion organoid model
hiPSCs (human induced pluripotent stem cells): human induced pluripotent stem cells; iNPC (derived neural progenitor cell): induced neural progenitor cell; iEC (derived endothelial cell): induced endothelial cell; MSC (mesenchymal stem cell): mesenchymal stem cell
In the fusion experiments, researchers carefully adjusted the culture medium components and found that 5% Geltrex matrix gel and 0.025 wt% hyaluronic acid significantly promoted spheroid fusion, while high concentrations of ROCK inhibitor Y27632 hindered the fusion process. Through a series of assessment methods, including cell proliferation, metabolic activity detection, cytokine secretion analysis, expression detection of neural and vascular markers, and gene expression analysis, researchers observed that the fused spheroids not only successfully promoted the differentiation of neuronal and vascular cells but also exhibited significantly upregulated gene expression levels related to brain region-specific markers, matrix remodeling, and BBB characteristics.
Moreover, electrophysiological property tests further confirmed that the cells derived from the fused spheroids possess neuronal functions and morphological characteristics. Compared to traditional direct mixing methods, the spheroid fusion strategy demonstrated significant advantages. This strategy avoids the process of cell separation and recombination, reducing cell loss while allowing the creation of mixed spheroid structures with carefully designed compartments, thus providing researchers with more precise experimental control. Additionally, this precise arrangement promotes the secretion of VEGF-A by assembling cortical spheroids, vascular spheroids, and MSCs, accelerating cortical tissue development and exhibiting potential for layered distribution.
1.2.3 Bioengineering Techniques
Although many in vitro culture models exhibit branching blood vessels and their perfusion capabilities have been predicted or confirmed, these models generally share a common flaw: the lack of active blood flow. Furthermore, there are varying degrees of limitations in precision, reproducibility, functional simulation, and experimental application. Bioengineering technologies, such as three-dimensional (3D) printing technology and microfluidic chip technology, have emerged and developed, bringing new hope and possibilities to overcome these limitations[18].
Microfluidic chip technology involves the precise manipulation of fluids at the micron scale, integrating biological, chemical, and medical analysis processes, with advantages such as small volume, fast speed, low consumption, and low cost, making it suitable for precise control of microenvironments and cell co-culture in organoid vascularization research[18]. Related studies designed and manufactured a microfluidic chip with an “open well” structure using 3D printing technology, selecting Dental SG resin as the printing material[19]. Through a special cleaning procedure, the biocompatibility of the chip was ensured. The center of the chip is the organoid culture chamber, with microfluidic channels on both sides, connected by a 50 μm gap, allowing for the exchange of cells and molecules.
Both vascular cells and brain organoids are derived from human pluripotent stem cells (hPSCs). On day 6 of differentiation, vascular cells were seeded into the microfluidic channels, while brain organoids were seeded into the organoid culture chamber on day 5, achieving co-culture of the two. Under the induction of a VEGF-A-containing culture medium, vascular cells extended vascular sprouts from the microfluidic channels into the organoid culture chamber, forming an ordered vascular network that interacted with the brain organoids, ultimately resulting in integrated neurovascular organoids. Co-culture experimental results indicated that the presence of vascular cells may accelerate the maturation process of brain organoids. Furthermore, the generated vascular network was not only structurally intact but also functional, capable of small molecule perfusion and permeability experiments.
Compared to in vivo transplantation models, various in vitro organoid models offer a series of advantages, including strong control over environmental variables, low cost, high flexibility and reproducibility, and fewer ethical issues. However, in vitro models cannot fully replicate the complex physiological and biological conditions in vivo, and while they can simulate cell populations and intercellular interactions by culturing different types of cells, their behavior may differ from that in the complex tissue environment in vivo, leading to deviations in experimental results. For vascularized brain organoids cultured in vitro for extended periods, ensuring sustained growth and functional stability of the tissue remains a technical challenge. Various bioengineering technologies show potential to address these issues, but their application is still not mature and requires further in-depth research.
2
Drug Development Based on Vascularized Brain Organoids
The BBB is a key barrier that maintains homeostasis in the central nervous system, and its permeability regulation directly relates to the efficiency of drug delivery across the barrier and its efficacy in brain tissue, making it an important factor in central nervous system drug research. Vascularized brain organoids partially replicate the structure and function of the BBB, providing a new platform for drug development in the central nervous system. Saglam-Metiner et al.[8] introduced an ICU patient-on-a-chip platform that combines mast cells differentiated from hiPSCs with brain organoids, cultured in a three-dimensional matrix, successfully simulating the interaction between the BBB and brain tissue. This platform utilizes a membrane covered with human brain microvascular ECs to simulate the separation of the vascular lumen and tissue chamber, partially replicating the structure and function of the BBB. The study found that isoflurane could activate mast cells, microglia, and astrocytes, thereby increasing the expression of a series of pro-inflammatory genes. Additionally, there was an increase in the expression levels of gap junction proteins, inhibitory neurotransmitter receptors, and excitatory neurotransmitter receptors in brain tissue. This indicates that isoflurane may affect neuronal function and survival by triggering neuroinflammatory responses and altering neurotransmitter balance.
In contrast, midazolam has a more pronounced destructive effect on the BBB, as it can lower the resistance between ECs, increase barrier permeability, and damage barrier integrity. Furthermore, midazolam can activate mast cells and microglia, exacerbating glutamate-related neurotoxicity and inducing excessive expression of inflammatory factors such as interleukins and interferons, ultimately leading to a significant increase in apoptosis of brain tissue cells. Midazolam also inhibits the expression of inhibitory neurotransmitter receptors and excitatory neurotransmitter receptors, further disrupting neurotransmitter balance and causing significant negative effects on brain tissue function.
Compared to traditional research models, the combination of vascularized brain organoid technology with microfluidic platforms is closer to the physiological environment of the human brain, allowing for a more accurate reflection of the mechanisms of drug action in the brain, providing a powerful tool for central nervous system drug research, and helping to advance related drug development efforts. However, the BBB does not exist in isolation; it is maintained by the joint action of ECs, astrocytes, smooth muscle cells, and other surrounding cells. Simulating the interactions between these surrounding cells and ECs is equally important for replicating BBB function. How to further reproduce the tight junction structure and selective permeability of drug molecules in vitro models is a key focus of subsequent research.
3
Exploration of Mechanisms of Cerebrovascular Diseases
Brain organoid models offer unique advantages in accurately simulating the three-dimensional structure of human tissues and the complex interactions between cells, providing a unique advantage for in-depth exploration of disease mechanisms[20]. Recently, a study utilized brain organoid chips to provide new perspectives and insights into the pathogenesis of hereditary hemorrhagic telangiectasia (HHT) type 1[7]. HHT is a hereditary disease characterized by vascular fragility, with HHT type 1 caused by mutations in the ENG (ENDOGLIN) gene. The study obtained cells from a rare chimeric HHT type 1 patient, successfully generating homologous mutant and normal hiPSCs, and further differentiating them into ECs to simulate the vascular conditions of HHT type 1 in vitro. The results showed that under two-dimensional culture conditions, the functional performance of mutant and normal ECs was similar. However, when placed in a three-dimensional model, the vascular networks formed by mutant ECs exhibited numerous tissue defects: significantly reduced vascular density, smaller diameters, decreased EC numbers, and significantly increased vascular leakage. Additionally, the interaction between mutant ECs and pericytes was also impaired, with a significant decrease in pericyte coverage and increased distance from ECs, further exacerbating vascular fragility. Fluorescent dextran leakage experiments confirmed that the leakage degree of mutant vessels was higher, reflecting significant impairment of their barrier function.
Moreover, another study successfully replicated the characteristics of cerebral cavernous malformations (CCM) using patient-derived organoids, providing important insights into the pathogenesis of CCM[21]. CCM is a hereditary cerebrovascular disease characterized by clusters of numerous thin-walled blood vessels that are prone to rupture and bleeding, leading to stroke and neurological dysfunction. The study observed that the endothelial channels in CCM patient-derived BBB organoids were abnormally enlarged and arranged back-to-back, a phenomenon highly similar to the pathological features of CCM in vivo. Further analysis showed that the expression of tight junction proteins and basement membrane proteins was significantly reduced in these organoids, indicating that the integrity of the BBB was compromised. Single-cell RNA sequencing revealed significant upregulation of pathological marker genes in ECs and enhanced expression of angiogenesis-related genes, indicating that the pathological changes in ECs play a dominant role in the pathogenesis of CCM. Additionally, the study found that the potential of MSCs from CCM patients to differentiate into vascular smooth muscle cells was weakened, and this loss of smooth muscle cell development may be one of the key factors leading to the onset of CCM.
4
New Strategies for Treating Cerebrovascular Diseases
Organoid technology, as an emerging treatment strategy for cerebrovascular diseases, is showing significant development potential. In 2020, a study first explored the feasibility and potential mechanisms of brain organoid technology in the treatment of ischemic stroke[22]. Researchers transplanted brain organoids cultured for 55 days into a rat middle cerebral artery occlusion model, finding that the transplantation of brain organoids not only significantly reduced the volume of cerebral infarction but also significantly improved neurological function, an effect closely related to the multi-lineage differentiation ability of the brain organoids. The transplanted brain organoids could simulate in vivo cortical development, differentiating into various cell types, including neural progenitor cells, neurons, and astrocytes, promoting brain injury repair through neurogenesis, synaptic reconstruction, axonal regeneration, and angiogenesis. Additionally, the transplanted cells could migrate extensively along the corpus callosum and survive for extended periods in the damaged area.
The study also indicated that transplanting organoids within 6 or 24 hours post-stroke significantly alleviated brain damage and improved behavioral performance, while transplantation beyond 7 days had limited effects, providing important evidence for brain organoids as a novel treatment strategy for ischemic stroke. Subsequently, another study further explored this field, particularly the role of brain organoids in long-term tissue repair and functional recovery[23]. Researchers induced brain organoids from hPSCs and transplanted them into the junction between the infarct core and the surrounding area in NOD-SCID mice. Months later, the organoids could survive long-term within the infarct core, differentiating into target neurons and forming functional synapses connected to the host neural network, restoring the structure of the damaged brain tissue.
The transplanted organoids differentiated into mature glutamatergic neurons and extended axons to distal brain regions, integrating into the host neural circuits. This structural repair and functional integration were reflected in improved mouse behavior, including recovery of sensory and motor functions. Compared to single-cell transplantation, brain organoid transplantation exhibited higher cell survival rates, stronger tissue repair capabilities, and superior neurological functional recovery effects. This study refined the implementation methods for brain organoid transplantation, demonstrating its ability to reconstruct infarcted tissue and restore post-stroke functional impairments. Thus, brain organoids open a highly promising new avenue for the treatment of cerebrovascular diseases, with exciting prospects for their application in ischemic stroke treatment.
5
Summary and Outlook
Organoid technology opens new perspectives for the research and treatment of cerebrovascular diseases, demonstrating broad potential in exploring disease mechanisms, drug development, and treatment strategies. Through innovations in in vivo transplantation, in vitro culture, and bioengineering technologies, the construction of vascularized brain organoids gradually overcomes the structural and functional limitations of traditional models, providing a more physiologically relevant experimental platform for studying neurovascular unit interactions, angiogenesis, and BBB function. Furthermore, these models achieve precise assessment of central nervous system drug effects in drug screening, providing breakthrough methods for studying the pathogenesis of complex cerebrovascular diseases and showing significant potential in treatment strategies.
Despite the progress made in organoid technology, many challenges remain. Ethical issues and safety assessments are key concerns in its clinical application, especially regarding human stem cells and gene editing technologies. The use of human stem cells (especially embryonic stem cells) raises ethical issues due to their origin from embryos, which may lead to the termination of life during embryonic development. To ensure ethical compliance, all research involving human stem cells must strictly adhere to international ethical standards, ensuring informed consent from research participants and guaranteeing data transparency. Additionally, gene editing technologies (such as CRISPR/Cas9), while providing powerful tools for organoid research, also carry ethical issues and safety risks, particularly when editing germ cells or embryos, which may raise ethical controversies regarding designer babies and have uncertain impacts on the genetic information of future generations. Therefore, the clinical application of gene editing technologies must undergo rigorous ethical review and ensure that the editing process does not produce unforeseen consequences for patients and their descendants. All gene editing operations should follow the principle of “minimal intervention,” making precise corrections only to necessary genes.
In terms of safety assessment, the clinical translation of organoid technology faces challenges such as cellular heterogeneity, immune responses, and the risk of tumorigenesis. The heterogeneity of organoids during in vitro culture may lead to differences in effects among different patients. Therefore, rigorous animal experiments and long-term safety testing are required to assess the clinical application risks. Furthermore, organoid transplantation may trigger immune rejection responses and cancer risks, which need to be further validated through experimental data, and detailed safety assessment standards should be established.
In terms of technical implementation, in vitro organoid models have advantages in controlling environmental variables, reducing costs, and addressing ethical issues, but they struggle to fully replicate the complex physiological environment and hemodynamics in vivo, and maintaining functional stability during long-term culture remains a challenge. In vivo transplantation models, while compensating for this deficiency, have vascular components derived from rodents, limiting their clinical application in humans. Additionally, the maturity and vascular function of these models still differ, and further optimization of vascularization efficiency and active perfusion capabilities is urgently needed[18]. The simulation of the BBB also faces challenges, particularly in effectively reproducing tight junctions and drug permeability, necessitating further research and technological breakthroughs.
Combining cutting-edge methods such as gene editing, microfluidic technology, and high-throughput 3D printing, there is hope for further enhancing the precision and functionality of organoid models in the future. Particularly in the field of cerebrovascular diseases, exploring the application potential of vascularized brain organoids in personalized treatment, such as designing customized drug screening platforms and treatment plans for specific patients, will promote the development of precision medicine. Meanwhile, with the advancement of bioengineering technologies and the deepening of interdisciplinary collaboration, organoid technology is expected to gradually achieve translation from the laboratory to clinical practice[24], bringing revolutionary breakthroughs to the diagnosis and treatment of cerebrovascular diseases.
(This article was edited by Li Yule)


References
[1] Walter K. What is acute ischemic stroke? [J]. JAMA, 2022, 327(9): 885.
[2] Montaño A, Hanley D F, Hemphill J C, 3rd. Hemorrhagic stroke [J]. Handb Clin Neurol, 2021, 176: 229-248.
[3] Goldstein L B. Introduction for focused updates in cerebrovascular disease [J]. Stroke, 2020, 51(3): 708-710.
[4] Li Y, Zeng P M, Wu J, et al. Advances and applications of brain organoids [J]. Neurosci Bull, 2023, 39(11): 1703-1716.
[5] Sato T, Vries R G, Snippert H J, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche [J]. Nature, 2009, 459(7244): 262-265.
[6] Song L Q, Yan Y W, Marzano M, et al. Studying heterotypic cell-cell interactions in the human brain using pluripotent stem cell models for neurodegeneration [J]. Cells, 2019, 8(4): 299.
[7] Orlova V V, Nahon D M, Cochrane A, et al. Vascular defects associated with hereditary hemorrhagic telangiectasia revealed in patient-derived isogenic iPSCs in 3D vessels on chip [J]. Stem Cell Reports, 2022, 17(7): 1536-1545.
[8] Saglam-Metiner P, Yanasik S, Odabasi Y C, et al. ICU patient-on-a-chip emulating orchestration of mast cells and cerebral organoids in neuroinflammation [J]. Commun Biol, 2024, 7(1): 1627.
[9] Hofer M, Lutolf M P. Engineering organoids [J]. Nat Rev Mater, 2021, 6(5): 402-420.
[10] Mansour A A, Gonçalves J T, Bloyd C W, et al. An in vivo model of functional and vascularized human brain organoids [J]. Nat Biotechnol, 2018, 36(5): 432-441.
[11] Wilson M N, Thunemann M, Liu X, et al. Multimodal monitoring of human cortical organoids implanted in mice reveal functional connection with visual cortex [J]. Nat Commun, 2022, 13(1): 7945.
[12] Pham M T, Pollock K M, Rose M D, et al. Generation of human vascularized brain organoids [J]. Neuroreport, 2018, 29(7): 588-593.
[13] Kistemaker L, van Bodegraven EJ, de Vries HE, Hol EM. Vascularized human brain organoids: current possibilities and prospects [J]. Trends Biotechnol, 2025, S0167-7799(24)00357-3.doi: 10.1016/j.tibtech.2024.11.021. Epub ahead of print.
[14] Cakir B, Xiang Y F, Tanaka Y, et al. Engineering of human brain organoids with a functional vascular-like system [J]. Nat Methods, 2019, 16(11): 1169-1175.
[15] Wörsdörfer P, Dalda N, Kern A, et al. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells [J]. Sci Rep, 2019, 9(1): 15663.
[16] Shi Y C, Sun L, Wang M D, et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo [J]. PLoS Biol, 2020, 18(5): e3000705.
[17] Song L Q, Yuan X G, Jones Z, et al. Assembly of human stem cell-derived cortical spheroids and vascular spheroids to model 3-D brain-like tissues [J]. Sci Rep, 2019, 9(1): 5977.
[18] Tan S Y, Feng X H, Cheng L K W, et al. Vascularized human brain organoid on-chip [J]. Lab Chip, 2023, 23(12): 2693-2709.
[19] Salmon I, Grebenyuk S, Abdel Fattah A R, et al. Engineering neurovascular organoids with 3D printed microfluidic chips [J]. Lab Chip, 2022, 22(8): 1615-1629.
[20] Xu C, Alameri A, Leong W, et al. Multiscale engineering of brain organoids for disease modeling [J]. Adv Drug Deliv Rev, 2024, 210: 115344.
[21] Dao L, You Z, Lu L, et al. Modeling blood-brain barrier formation and cerebral cavernous malformations in human PSC-derived organoids [J]. Cell Stem Cell, 2024, 31(6): 818-833.e11.
[22] Wang S N, Wang Z, Xu T Y, et al. Cerebral organoids repair ischemic stroke brain injury [J]. Transl Stroke Res, 2020, 11(5): 983-1000.
[23] Cao S Y, Yang D, Huang Z Q, et al. Cerebral organoids transplantation repairs infarcted cortex and restores impaired function after stroke [J]. NPJ Regen Med, 2023, 8(1): 27.
[24] Zheng F Y, Xiao Y M H, Liu H, et al. Patient-specific organoid and organ-on-a-chip: 3D cell-culture meets 3D printing and numerical simulation [J]. Adv Biol (Weinh), 2021, 5(6): e2000024.
Author Contributions
Xue Jiaohao was responsible for writing the paper and creating images; Li Zhipeng was responsible for topic design and literature search; Zhao Yuanli coordinated team member assignments, advanced writing progress, and reviewed the paper.
First Author

Beijing Tiantan Hospital
Xue Jiaohao
Physician, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University.
Research direction: cerebrovascular diseases, collaborative prevention and treatment of brain-heart comorbidities.

Beijing Tiantan Hospital
Li Zhipeng
Physician, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University.
Research direction: surgical treatment and hemodynamic research of cerebrovascular diseases.
Corresponding Author

Beijing Union Medical College Hospital
Zhao Yuanli
Director of Neurosurgery, Chief Physician, Professor, Doctoral Supervisor. Editor-in-Chief of the Chinese Journal of Neurosurgery (English Edition), Member of the Chinese Medical Association Neurosurgery Branch.
He has hosted or participated in multiple national and provincial-level major scientific research projects, including the National Natural Science Foundation, National Key Research and Development Program, National Key Technology Support Program, and National Clinical Medical Research Center for Neurological Diseases. He has participated in several national key scientific research projects on cerebrovascular diseases and cranial tumors, receiving multiple awards including the Second Prize of National Science and Technology Progress, First Prize of Beijing Science and Technology Progress, First Prize of Chinese Medical Science and Technology, and the Ma Yisheng Youth Science and Technology Award.
In 2020, he was selected as a national-level candidate for the Hundred-Thousand-Ten Thousand Talent Project and recognized as a national-level expert with outstanding contributions. He has published over 80 SCI papers and edited several books. His main research focuses on clinical and basic research of complex cerebrovascular diseases and brain tumors, specializing in the surgical treatment of complex cerebral aneurysms, vascular malformations, cerebral hemorrhage, moyamoya disease, and other cerebrovascular diseases, as well as surgical treatment of various cranial and spinal tumors, brain injuries, and functional neurological diseases, and repair and reconstruction of nerve injuries.

Conference Notice


Participants wishing to register for the conference should do so through the conference website, registration link: https://cap2025.sciconf.cn/

Editor 丨Liu Yang, Zhao Na
Proofreader 丨Li Na, Li Yule
Supervisor 丨Peng Bin

Founded in 2010, the journal is supervised by the National Health Commission and hosted by Peking Union Medical College Hospital, a comprehensive medical journal that has been rated as a “Core Journal of Chinese Science and Technology,” “Source Journal of the Chinese Science Citation Database (CSCD),” and “Core Journal Overview of Chinese Core Journals (Peking University Chinese Core).” It is indexed in Scopus and DOAJ databases and included in the “Excellent Action Plan for Chinese Science and Technology Journals” project.

The Chinese Journal of Medicine advocates respect for and protection of intellectual property rights. Reproduction and citation are welcome, but authorization from this platform is required. If you have any doubts about the content and copyright of the article, please send an email to [email protected], and we will communicate and handle it promptly. The content is for communication and learning purposes only and is not for profit; popular science content is only for public health knowledge dissemination, and readers should not use it as a basis for individual diagnosis and treatment, to avoid delaying treatment. For medical needs, please consult online or offline through the Beijing Union Medical College Hospital APP.