Click the blue text to follow us
Overview of the full text
This study uses gadolinium borate, which has a simple chemical formula and is easy to synthesize, as the matrix, selecting Ce3+, Tb3+, and Eu3+ with different luminescent properties as the doped ions. The aim is to prepare rare earth-doped GdBO3 luminescent materials at both nano and non-nano scales through different synthesis methods, to study the differences in luminescent performance between nano and non-nano materials, and to optimize the most practically valuable synthesis scheme. For conventional (non-nano) samples, we use rare earth oxides as reactants and synthesize them using a high-temperature solid-state method; for nano samples, we use rare earth nitrates as reactants and synthesize them using a hydrothermal method. Subsequently, we characterize the phase purity of the obtained samples using X-ray powder diffraction technology and measure their photoluminescent properties.
Innovative Points
LEDs are the most valuable new generation light sources of the 21st century, possessing excellent properties such as energy saving, environmental protection, and long lifespan. Therefore, the complete replacement of traditional light sources is the trend of future social development. Borates are inexpensive, chemically stable, and exhibit excellent luminescent properties after rare earth ion doping, making them of significant research value in the field of phosphors for converting white light LEDs. By utilizing Ce3+, Tb3+, and Eu3+ as luminescent centers, the research on synthesizing red, green, and blue phosphors is relatively mature. However, whether co-doping Ce3+, Tb3+, and Eu3+ in the same matrix can achieve a wide range of control over color and color temperature in tricolor phosphors still has considerable research space. Energy transfer is a common phenomenon in the field of luminescence; controlling the energy transfer rate between different luminescent centers can change the luminescent properties of the luminescent materials.Ce(III), Tb(III), and Eu(III) are important activators in traditional tricolor phosphors, capable of achieving relatively ideal blue, green, and red light emission in different matrices. When in the same matrix, Ce(III) often sensitizes Tb(III), and Eu(III) can often be activated by Tb(III), thus deriving an energy transfer chain starting from Ce(III), mediated by Tb(III), and ending at Eu(III). By achieving energy transfer of Ce-Tb-Eu in the GdBO3 matrix, we can control the final luminescent properties of the phosphors and adjust the emission spectrum of the phosphors.
Image Analysis
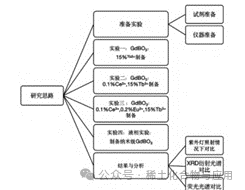
Figure 1: Experimental Flowchart
Experimental Design (See Figure 1) High-temperature solid-state method is a commonly used powder preparation process, although it has some inherent disadvantages, such as high energy consumption, low efficiency, high roughness of the powder, and susceptibility to contamination. However, due to its advantages of non-agglomerated powder particles, good packing properties, low cost, high yield, and simple preparation process, the high-temperature solid-state method is still widely used. The process includes two main stages: high-temperature reaction and solid-state transformation. In the high-temperature reaction stage, the raw materials react under high temperature, high pressure, and chemical reactions to produce reaction products. The key in this stage is to provide sufficient energy at high temperatures to induce chemical changes in the raw materials. High temperature and high pressure conditions favor the reaction, promoting dehydration, gas release, oxidation, and other reactions between the raw materials, thus generating new compounds or substances. These reaction products typically have high purity and specific crystalline structures, which can be adjusted and optimized as needed.
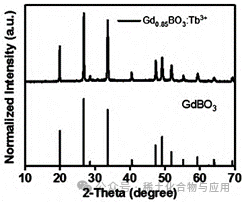
Figure 2: Completely Overlapping XRD Images
Figure 3: Comparison of Indoor Light and UV Light
This experiment successfully synthesized luminescent materials (GdBO3: 15% Tb3+), and through XRD image comparison, the synthesized product has an image completely overlapping with GdBO3 (See Figure 2), and the synthesized material emits weak light under UV light but appears white under normal indoor lighting (See Figure 3).
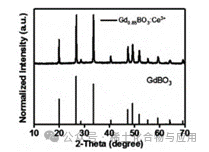
Figure 4: Completely Overlapping XRD Images
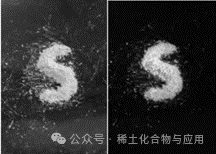
Figure 5: Comparison of Indoor Light and UV Light
Through XRD image comparison, the synthesized product has an image completely overlapping with GdBO3 (Figure 4), and the second product emits light under UV light significantly enhanced compared to the first product, showing a bluish-green color (Figure 5).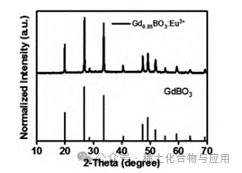
Figure 6: Completely Overlapping XRD Images
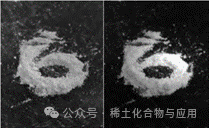
Figure 7: Comparison of Indoor Light and UV Light
This experiment successfully synthesized luminescent materials (GdBO3: 0.1% Ce3+, 15% Tb3+, 0.2% Eu3+), and through XRD image comparison, the synthesized product has an image completely overlapping with GdBO3 (Figure 6), and under UV light, it emits a faint yellow color (Figure 7).
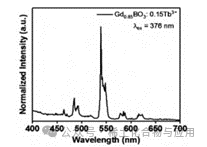
Figure 8: GdBO3: 15% Tb3+ Luminescent Spectrum
After scanning GdBO3: 15% Tb3+ with a fluorescence spectrometer, its emission spectrum was obtained (Figure 8). From this emission spectrum, it can be seen that the light emission of GdBO3: 15% Tb3+ tends to stabilize below a wavelength of 460nm, forming emission peaks at approximately 462, 481, 486, 540, 570, and 620nm. It can be inferred that this sample will be excited and emit green light under UV light with a wavelength of 380nm, demonstrating good optical performance.
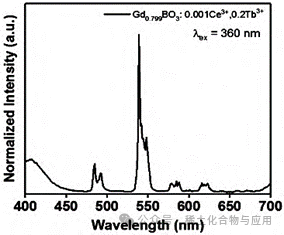
Figure 9: GdBO3: 15% Tb3+, 0.1% Ce3+ Emission Spectrum
Using a combination fluorescence spectrometer to scan GdBO3: 15% Tb3+, 0.1% Ce3+, the emission spectrum was obtained (Figure 9). In this emission spectrum, GdBO3: 15% Tb3+, 0.1% Ce3+ emits light at approximately 410, 475, 485, 540, 575, and 620nm, forming emission peaks. It can be concluded that this sample will be excited and emit strong bluish-green light under UV light with a wavelength of 380nm.
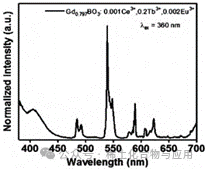
Figure 10: GdBO3: 15% Tb3+, 0.1% Ce3+, 0.2% Eu3+ Emission Spectrum
For GdBO3: 15% Tb3+, 0.1% Ce3+, 0.2% Eu3+, spectral detection was performed to obtain its emission spectrum (Figure 10). From this emission spectrum, it can be seen that GdBO3: 15% Tb3+, 0.1% Ce3+, 0.2% Eu3+ emits light at approximately 380, 410, 480, 490, 540, 577, 608, and 624nm, forming emission peaks. It can be concluded that this sample will be excited and emit a faint yellow light under UV light with a wavelength of 380nm.
Future Development
In the synthesis and application of rare earth-doped upconversion nanomaterials, we have conducted a lot of research work. However, the development of rare earth-doped upconversion nanomaterials is severely constrained by low luminescent efficiency. Under the same doping concentration of rare earth ions, bulk materials generally have higher luminescent efficiency. Therefore, finding the fundamental reasons for the differences in luminescent efficiency between bulk materials and nanomaterials will become a key direction for future research. Compared to rare earth-doped upconversion nanomaterials, bulk materials are usually prepared under high-temperature conditions. Therefore, we need to further explore whether high temperature is a key factor determining luminescent efficiency. These issues need to be discussed in subsequent research.
Currently, the preparation process of nanoluminescent materials still has imperfections, and the preparation process requires high standards. Therefore, improving the stability and reliability of nanoluminescent materials will become a research topic in the future. This will enable research results to be truly applied on a large scale in practical fields. Additionally, experiments have shown that co-doping different doses of Ce3+, Tb3+, and Eu3+ can make the samples emit different colors of visible light, all of which are bright and vivid. This can be used for multicolor biological labeling and the production of different colored light lamps. Therefore, studying the different colors that arise from varying the proportions of co-doped Ce3+, Tb3+, and Eu3+ will greatly aid future practical luminescent applications.
Journal Author
Guangdong Chemical,2024Issue 516
Authors: Bai Jialin, He Cheng, Wang Yue, et al.
Author Affiliation: Nanjing Police Academy
