 Synergizing Catalysis with Post-catalysis Pseudo-Iron Release by Building Dynamic Catalytic Active Sites in Diatomic Nanozymes for Boosting Cancer TherapyJournal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-04-25DOI: 10.1021/jacs.5c03804Diatomic nanozymes have significant potential in tumor therapy, but their therapeutic efficacy is often limited by their low catalytic activity. Here, we propose a Ga/Zn diatomic nanozyme (Ga/Zn-nc) with a well-defined geometric structure and electronic configuration, designed to mimic peroxidase and glutathione oxidase, exhibiting excellent catalytic activity for cascade catalysis. The formation of gallium-zinc metal bonds is essential for accelerating electron transfer and lowering the reaction energy barrier, thereby enhancing catalytic performance. In the tumor microenvironment, the catalytic action of Ga/Zn-NC induces oxidative damage, making breast cancer cells sensitive to ferroptosis. Meanwhile, the gallium released by Ga/Zn-NC with “pseudo-iron” activity disrupts iron metabolism, activating a self-amplifying ferroptosis pathway, which synergistically enhances ferroptosis and apoptosis, achieving significant anti-tumor efficacy.
Synergizing Catalysis with Post-catalysis Pseudo-Iron Release by Building Dynamic Catalytic Active Sites in Diatomic Nanozymes for Boosting Cancer TherapyJournal of the American Chemical Society ( IF 14.4 ) Pub Date : 2025-04-25DOI: 10.1021/jacs.5c03804Diatomic nanozymes have significant potential in tumor therapy, but their therapeutic efficacy is often limited by their low catalytic activity. Here, we propose a Ga/Zn diatomic nanozyme (Ga/Zn-nc) with a well-defined geometric structure and electronic configuration, designed to mimic peroxidase and glutathione oxidase, exhibiting excellent catalytic activity for cascade catalysis. The formation of gallium-zinc metal bonds is essential for accelerating electron transfer and lowering the reaction energy barrier, thereby enhancing catalytic performance. In the tumor microenvironment, the catalytic action of Ga/Zn-NC induces oxidative damage, making breast cancer cells sensitive to ferroptosis. Meanwhile, the gallium released by Ga/Zn-NC with “pseudo-iron” activity disrupts iron metabolism, activating a self-amplifying ferroptosis pathway, which synergistically enhances ferroptosis and apoptosis, achieving significant anti-tumor efficacy.
Introduction
In biological systems, nearly half of the enzymes catalyze reactions with the assistance of one or more metal ions, participating in countless biological processes including energy metabolism, DNA/protein synthesis, and cell signaling. (1-4) The metal ions in metalloenzymes help stabilize the tertiary structure of proteins, facilitate electron transfer, and/or serve as active sites for catalyzing various reactions. (4-6) These metal ions typically coordinate with donor ligands (such as S, O, N) in amino acid residues or cofactors (like protoporphyrin), forming a defined geometry and electronic structure that determines enzyme-substrate interactions. (7)
In recent years, artificially synthesized nanozymes have been developed to mimic natural enzymes, and in some cases, they can even generate new natural catalytic activities for therapeutic and other purposes. (8-11) Compared to natural enzymes, nanozymes offer advantages such as better stability, lower cost, ease of mass production, and diverse catalytic activities. (12,13) Various strategies have been explored to enhance the catalytic activity of nanozymes, including structural and electronic engineering, ligand functionalization, and the use of external stimuli. (13-17) Single-atom nanozymes with well-defined metal-ligand coordination exhibit outstanding catalytic activity due to their maximum atomic utilization. (18-20) Uniquely, they hold promise to mimic the electronic and structural geometries of metal-amino acid coordination in metalloenzymes, particularly those containing heteronuclear bimetallic centers with high intrinsic complexity. (14,21-24)
In heteronuclear metalloenzymes, two or more different metal ions are brought close together, coupling structurally and electronically, and acting synergistically. Zn(II) d-orbitals are filled, providing ligand field stabilization energy, forming the most stable ligand geometry to maintain the integrity of the protein structure, making it a natural evolutionary choice. (25,26) During catalysis, Zn(II) typically acts as a basic cofactor, not participating in redox reactions, but rather serving as a Lewis acid to accept a pair of electrons. (25,27-30)
In the parallel field of materials engineering, metal-organic frameworks (MOFs) using Zn2+ as metal nodes, such as zeolitic imidazolate frameworks-8 (ZIF-8), have been widely used as carriers for preparing single-atom catalysts and nanozymes. (31-33) However, in many reports, the role of zinc in the final single-atom products is often overlooked. Recently, some studies have emphasized the importance of zinc in regulating the redox potential of transition metals (Cu, Mn, Fe, Pt, Co) that act as catalytic centers. (37,38) By judiciously selecting second functional ions, nanozymes with efficient heteronuclear bimetallic active centers can be constructed.
Gallium is a heterometal with an ionic radius and chemical properties highly similar to iron. Ga(III) has been shown to bind with high affinity to a range of iron-binding proteins, disrupting intrinsic iron metabolism and iron-involved cellular processes. (39-42) Due to the high demand for iron in tumors, Ga(III) can actively concentrate in tumors, blocking iron uptake and metabolism, thereby inhibiting tumor cell proliferation, a phenomenon known as the “pseudo-iron” effect. Ga(III) compounds are currently in clinical trials for tumor therapy and are considered a new generation of metal-based drugs following platinum-based drugs. (43) However, their clinical application is limited by inefficient tumor targeting and off-target toxicity.
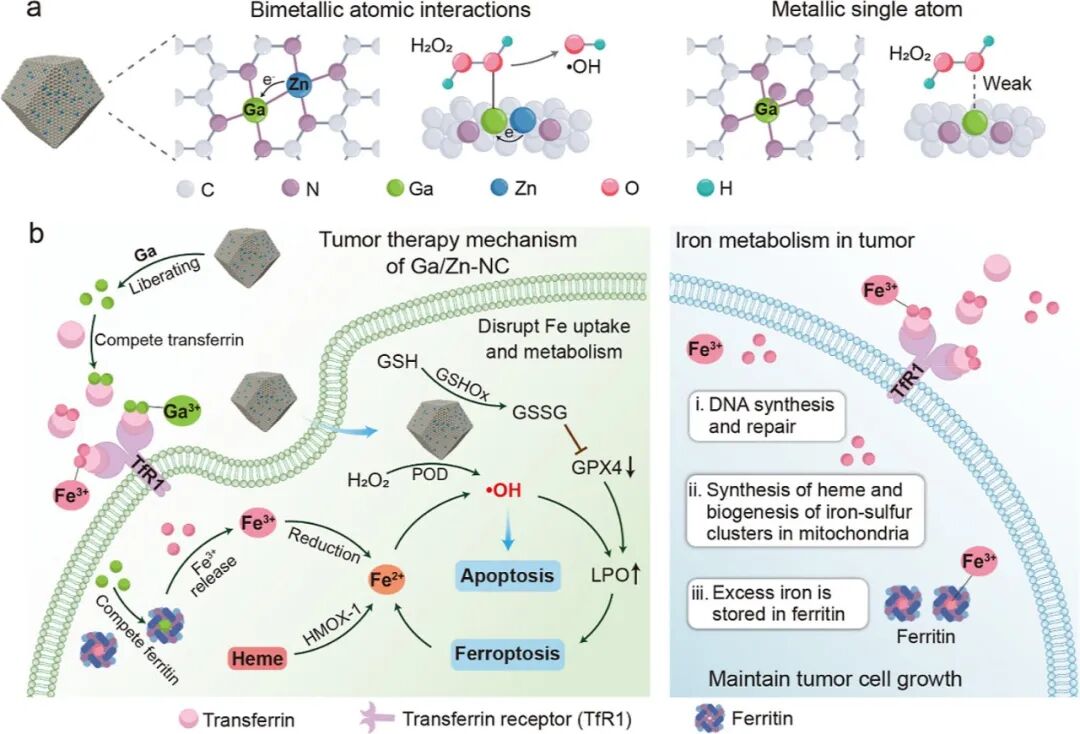

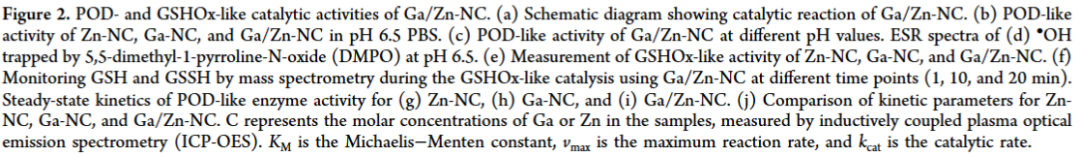
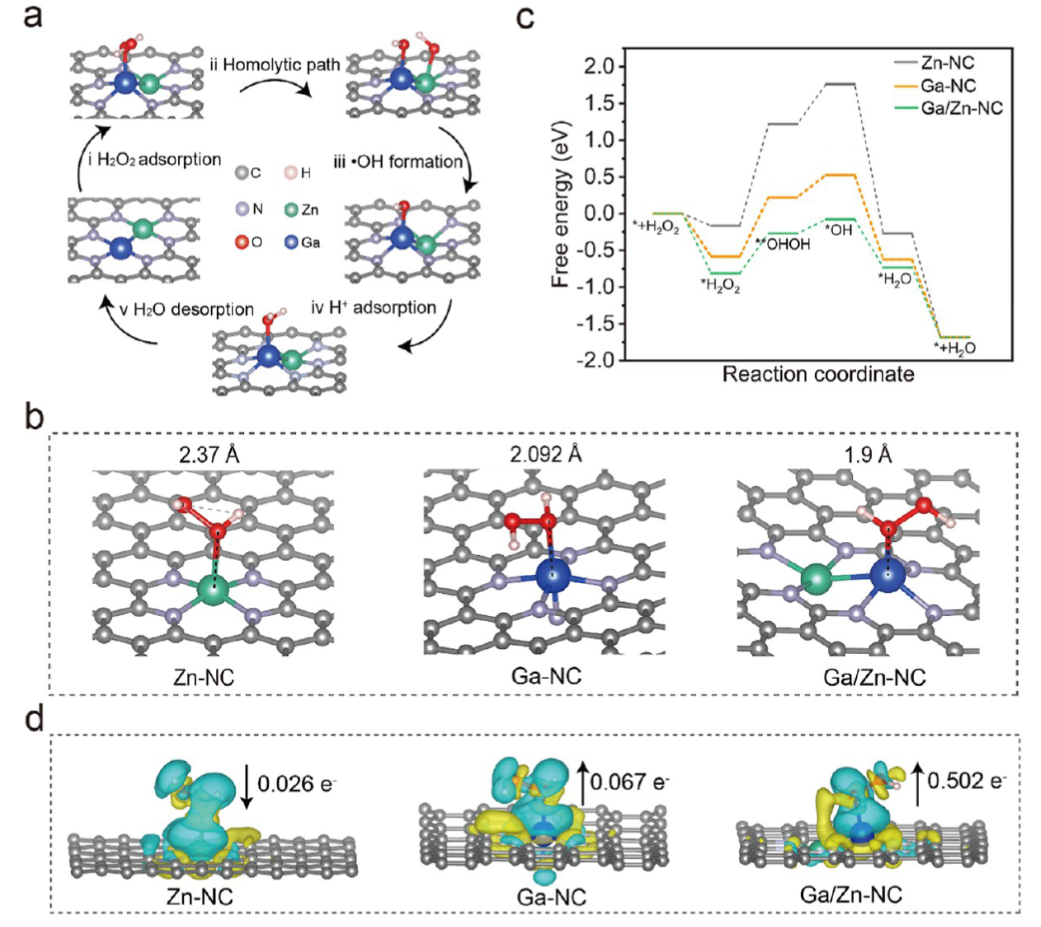
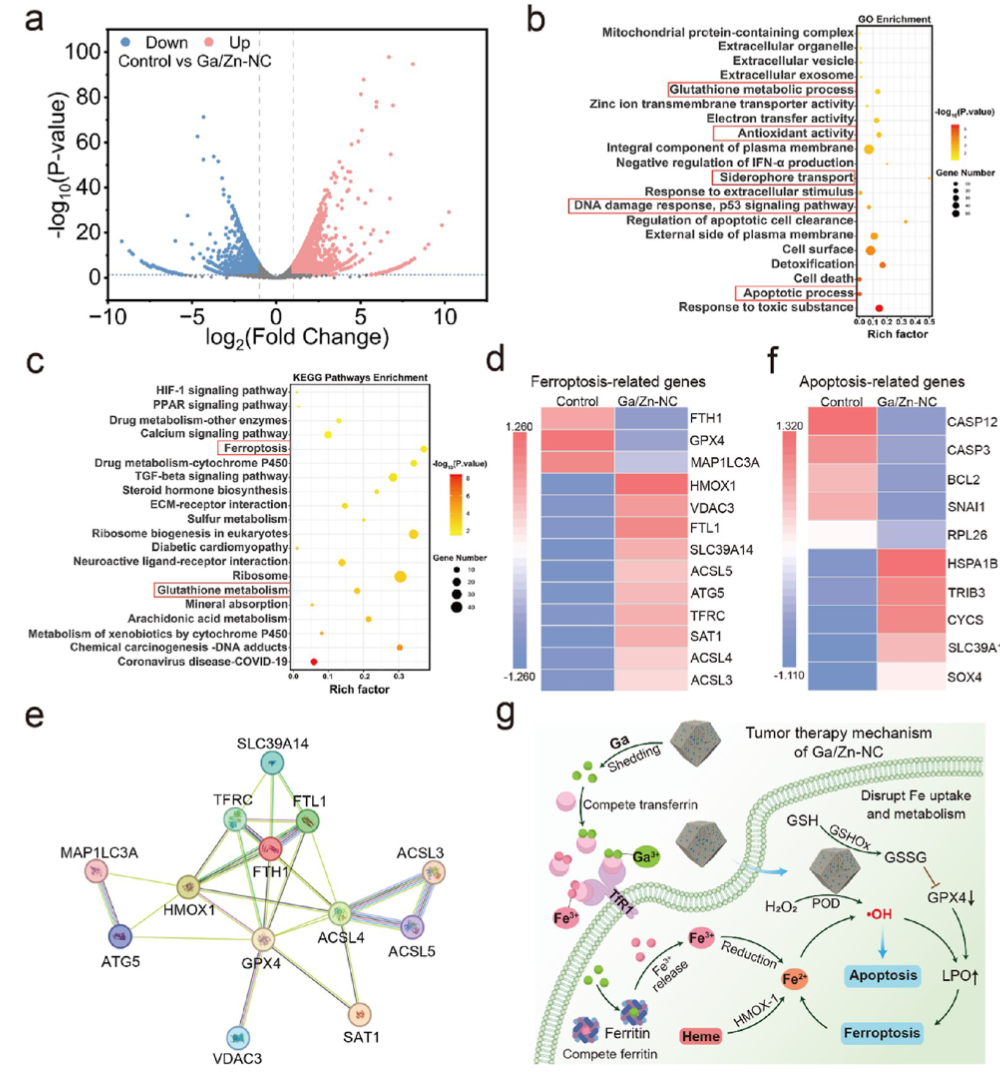
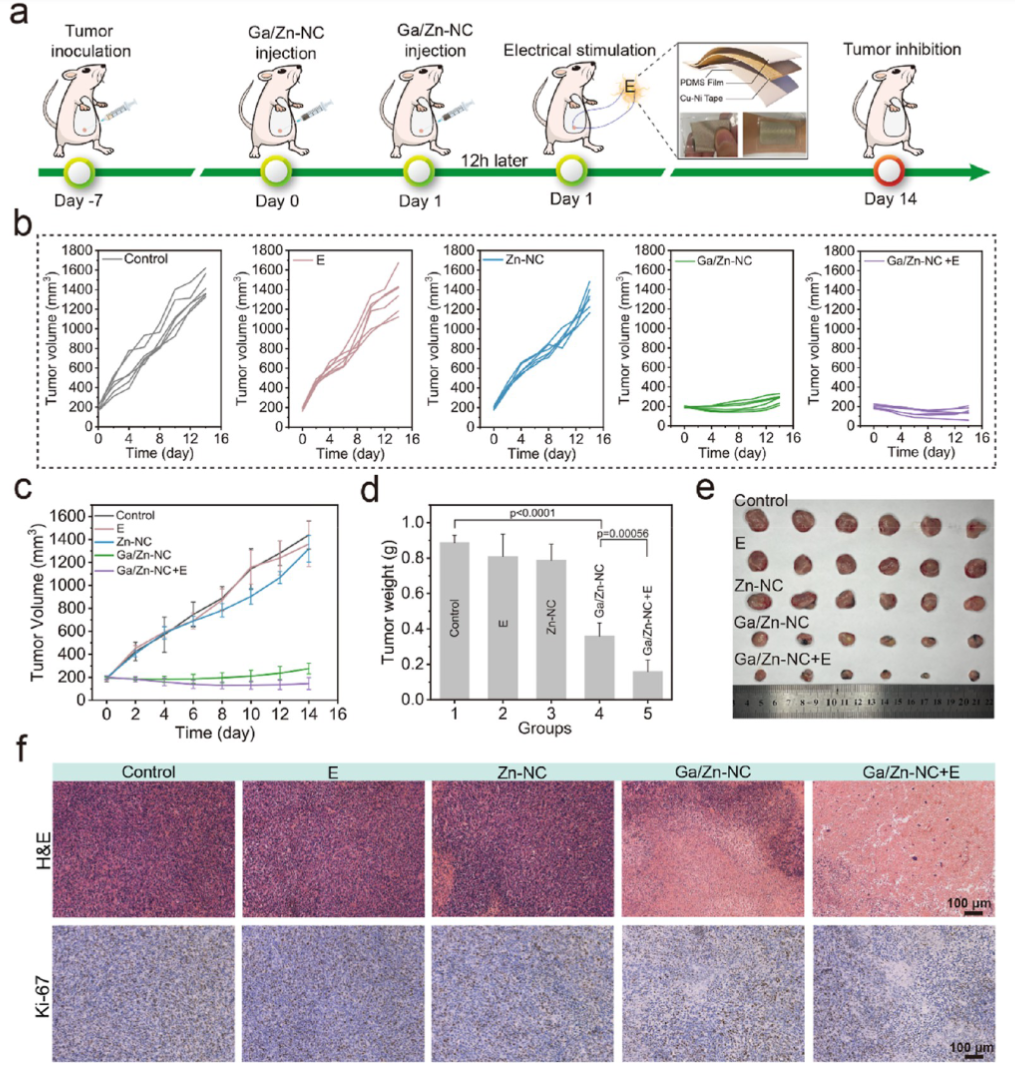
In this work, we propose an efficient Ga/Zn diatomic nanozyme (Ga/Zn-nc) with a well-defined structure and electronic configuration for cancer catalytic therapy (Scheme 1). Ga/Zn-nc mimics peroxidase (POD) and glutathione oxidase (GSHOx), catalyzing the generation of harmful hydroxyl radicals (•OH) and the consumption of the antioxidant glutathione (GSH), thereby directly killing tumor cells.
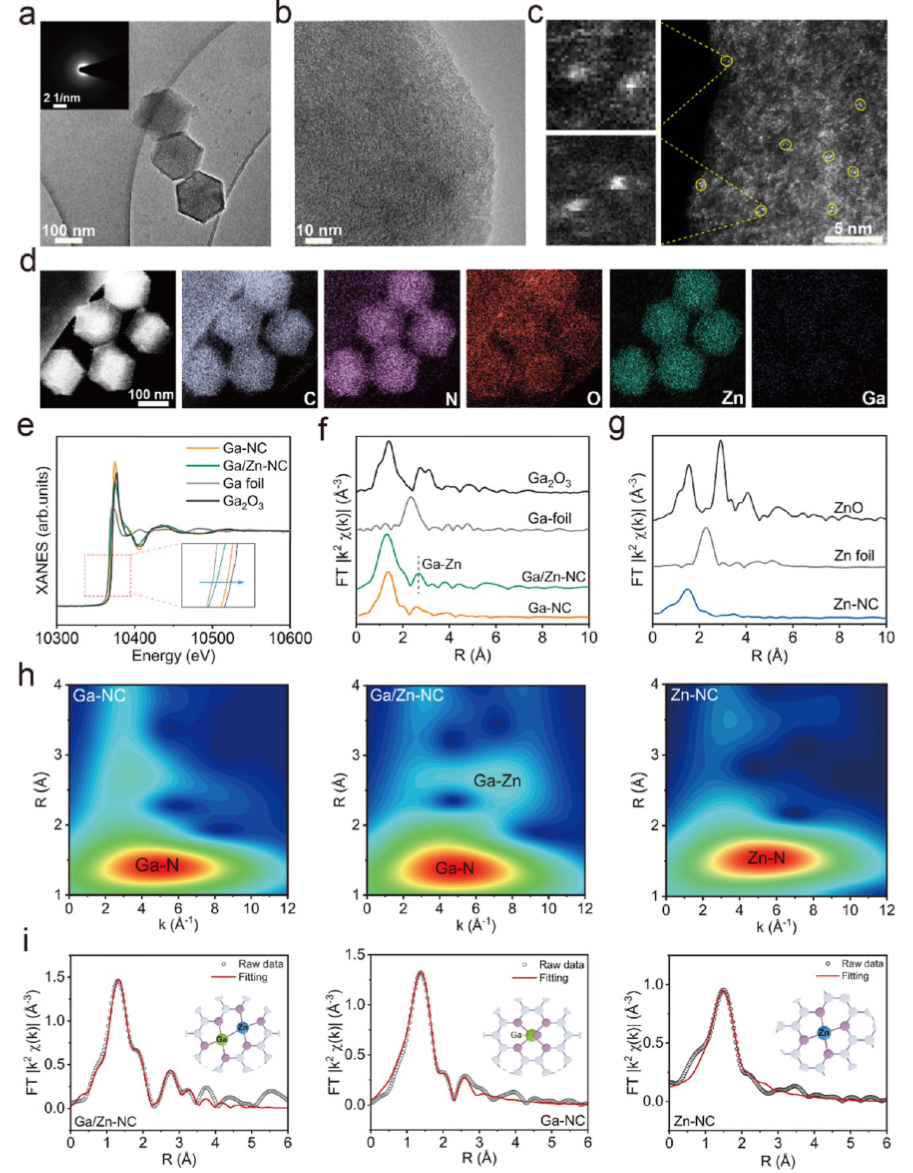
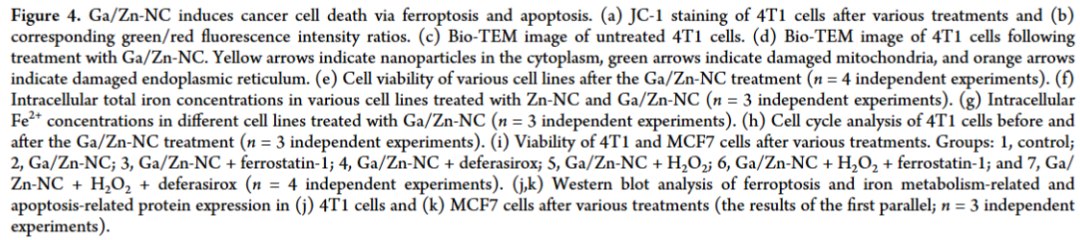
A single-atom nanozyme was constructed as a control, revealing that in the heteronuclear bimetallic-n coordination configuration, Ga serves as the primary active site, while Zn acts as a co-catalytic site to accelerate electron transfer during the kinetic transformation. Ga/Zn-NC synergistically enhances its catalytic activity, specifically releasing Ga(III) in the tumor microenvironment (TME), achieving gallium reutilization, enhancing ferroptosis, and amplifying catalytically induced cellular damage. Our work proposes a ready-to-use therapeutic strategy by establishing a self-amplifying loop of iron metabolism disorder and reactive damage loop.
Results and Discussion

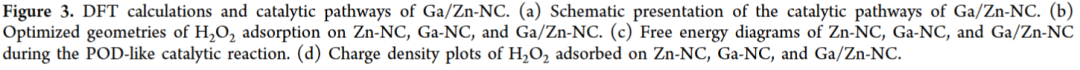

Conclusion
Artificial metalloenzymes characterized by heteronuclear bimetallic centers possess complex and variable structures that are difficult to replicate through novel biomimetic designs. Nanozymes represent a bottom-up approach, synthesized using simple ions and chemicals, providing a more economical, easily prepared, and stable alternative.
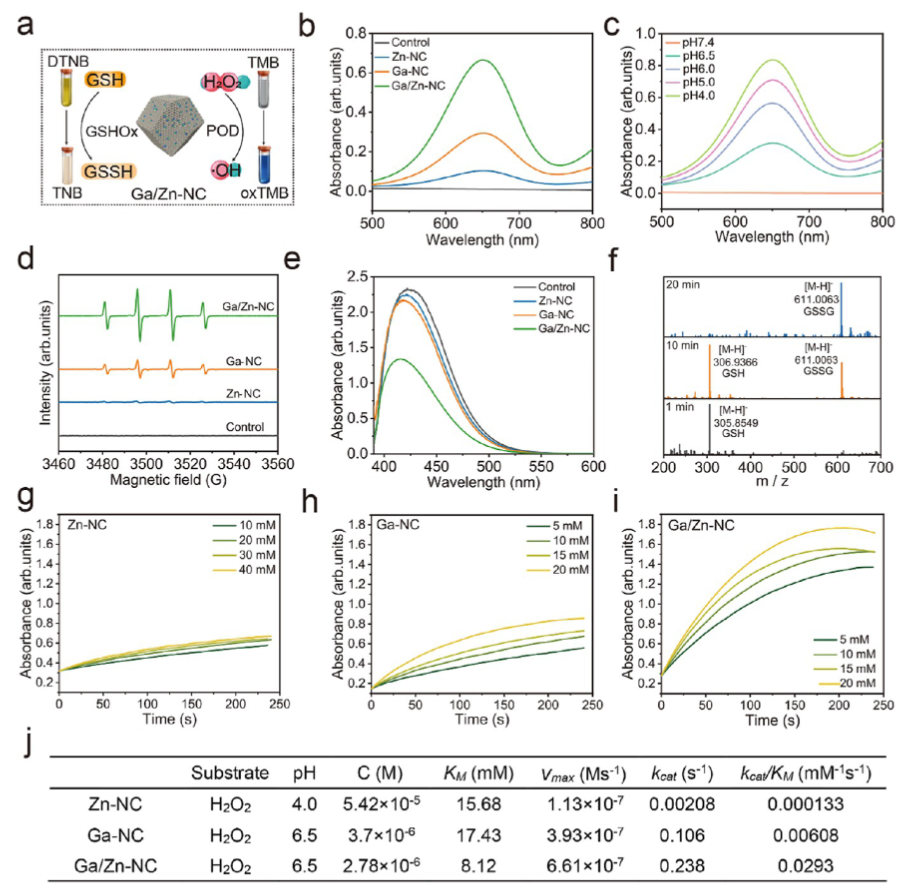
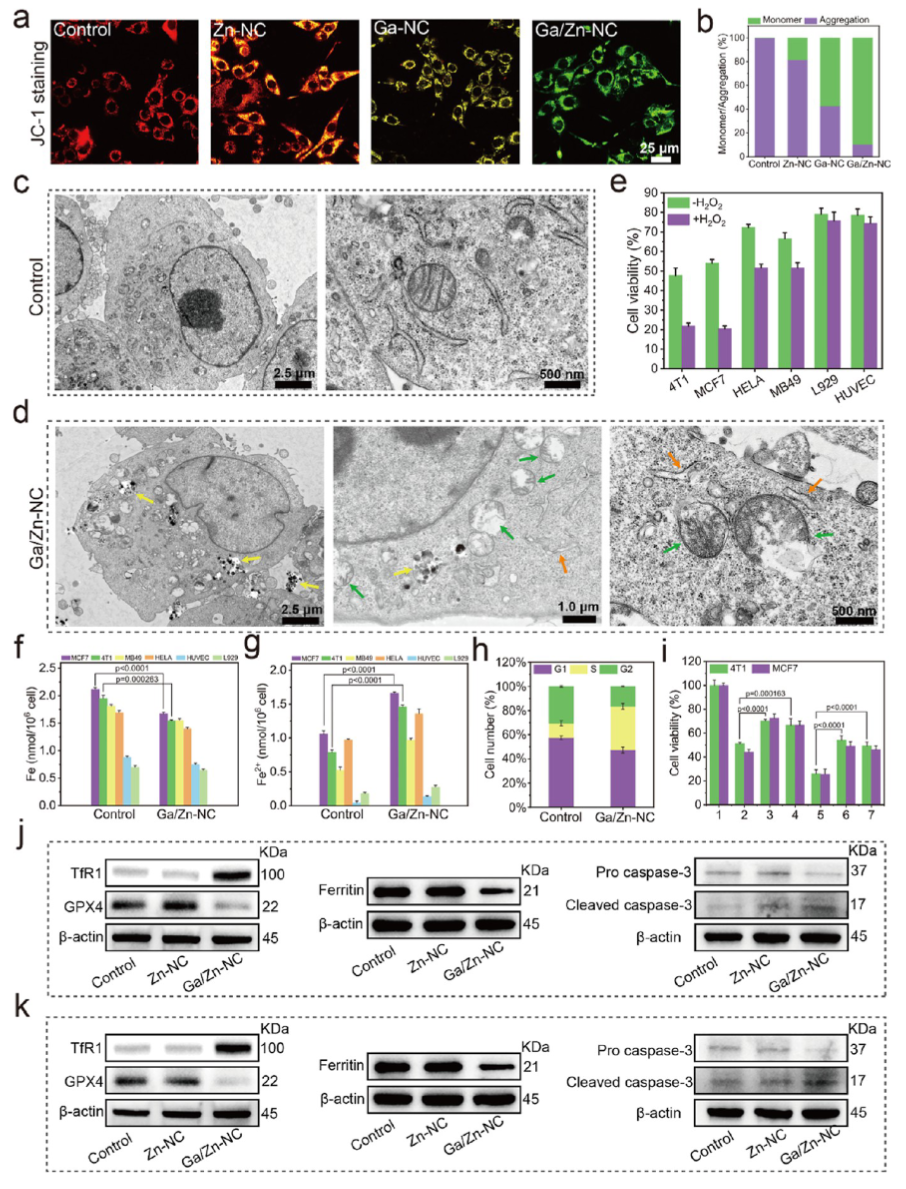
Ga/Zn diatomic nanozymes (Ga/Zn-nc) exhibit precisely defined metal-metal and metal-n coordination structures and electronic configurations, demonstrating extraordinary catalytic performance similar to that of POD and GSHOx functions. Kinetic analysis and DFT calculations provide compelling evidence that gallium-zinc metal bonding enhances substrate adsorption and dissociation.
In the diatomic configuration, Ga serves as the primary active site, while the adjacent Zn plays a supportive role, facilitating substrate activation on Ga, thereby accelerating the catalytic process. In tumors, the generation of •OH and the elimination of GSH are key events that sensitize cells to ferroptosis. Notably, in the TME, Ga/Zn-NC releases a portion of Ga(III) with a “pseudo-iron” effect, disrupting iron metabolism and activating a self-amplifying ferroptosis pathway, providing an additional advantage for TME treatment.
 ENDClickthe bluetext to jumpAdv.Matertumor therapysoftware collectionAngew.Chem.Int.Ed.biosensing & detectionOrigin 22J.Am.Chem.SochydrogelsPPT drawingChem.Eng.J.antibacterialJade 6.5Nat.Communanti-inflammatoryEndnote 21Chem.Soc. Rev.single atomresume templateNano Todayfunctional nanozymesPPT templateAnal. Chem.nanozyme reviewresearch essentialsjournal classificationapplication classificationresearch tools
ENDClickthe bluetext to jumpAdv.Matertumor therapysoftware collectionAngew.Chem.Int.Ed.biosensing & detectionOrigin 22J.Am.Chem.SochydrogelsPPT drawingChem.Eng.J.antibacterialJade 6.5Nat.Communanti-inflammatoryEndnote 21Chem.Soc. Rev.single atomresume templateNano Todayfunctional nanozymesPPT templateAnal. Chem.nanozyme reviewresearch essentialsjournal classificationapplication classificationresearch tools
●【Adv.Funct.Mater】Aggregation-Induced Emission Photosensitizers-Armored Magnetic Nanoparticles for Treating Sepsis: Combating Multidrug-Resistant Bacteria and Alleviating Inflammation
●【Small Methods】A Three-Pronged Approach of Mixed Nanoplatforms Including MXenes, Upconversion Nanoparticles, and Aggregation-Induced Emission Photosensitizers for Effective Cancer Therapy
●【Chem.Soc.Rev】Organic Photosensitizers for Antibacterial Phototherapy
●【Nano Today】Bonsai-Inspired Vermiculite Nanosheet-Based AIE Nanohybrid Photosensitizers for Ferroptosis-Assisted Self-Sufficient Photodynamic Cancer Therapy
●【Small Methods】A Three-Pronged Approach of Mixed Nanoplatforms Including MXenes, Upconversion Nanoparticles, and Aggregation-Induced Emission Photosensitizers for Effective Cancer Therapy
 For collaboration, please message the editor~
For collaboration, please message the editor~ Share
Share Collect
Collect View
View Like
Like