Research Background
Polyethylene terephthalate (PET) is a common plastic known for its high wear resistance/impact resistance, excellent chemical stability, and good transparency, widely used in food containers, packaging, clothing, carpets, ropes, and other fields. In 2022, the global production of PET reached 25.5 million tons, and it is expected to increase to 35.7 million tons by 2030. Meanwhile, the massive increase in PET waste poses a threat to the global environment and the ecological balance of nature, resulting in severe resource waste. Therefore, the recycling of PET waste has become particularly important today. Incineration and landfilling destroy the natural environment and waste a large amount of resources. Chemical recycling can achieve closed-loop recycling or upgraded recycling of PET, producing high-value-added products, including terephthalic acid, carbon nanomaterials (such as graphene, carbon foams, and porous carbon), and metal-organic frameworks (MOF). As a typical crystalline material, MOF is formed by coordination of metal nodes with organic bridging ligands, exhibiting excellent structural regularity, inherent porous networks, tunable component ratios at organic-inorganic interfaces, and numerous catalytically active coordination sites. Therefore, MOF has great potential in various applications such as gas adsorption and separation, water collection, heat redistribution, sensing, biomedical applications, and catalysis. Through various methods such as alcoholysis, hydrolysis, and mechanochemistry, waste PET can be decomposed into 1,4-benzenedicarboxylic acid (H2BDC), which is one of the most widely used organic linkers for preparing MOF. Therefore, the conversion of waste PET into MOF has become a promising research focus, offering dual advantages of valorizing plastic waste through advanced recycling strategies and economically synthesizing porous coordination polymers with tailored functionalities.
The current upcycling methods can be mainly divided into four types: two-step solvothermal method, one-step solvothermal method, microwave-assisted synthesis, and two-step ball milling method. In the two-step or one-step solvothermal methods, waste PET is first degraded into H2BDC, which then coordinates with metal clusters to form MOF, or PET is depolymerized into H2BDC and coordinated to synthesize MOF simultaneously, usually in the presence of organic solvents, which requires a large amount of toxic organic solvents (e.g., 100 mL DMF g-1 MOF), high temperatures (e.g., 150 ℃), and high pressures (e.g., 25 bar), and has disadvantages such as long reaction times (e.g., 24 h), environmental unfriendliness, high energy consumption, and production safety issues. The microwave-assisted synthesis method accelerates the degradation of PET and the crystallization of MOF by molecular agitation induced by electromagnetic fields. Compared to solvothermal methods, this technology has advantages such as enhanced reaction kinetics, shortened reaction times (usually 1-4 h), improved yields of MOF, and reduced by-products. However, the large-scale application of this method is limited by the requirement for specialized equipment and still faces the issue of high organic solvent consumption. Recently, we proposed a two-step ball milling strategy to convert waste PET into MOF. Under the action of ball milling and NaOH, PET is decomposed into Na2BDC, and then metal salts are added and milled again to obtain a series of BDC-based MOF. This method has advantages such as ambient pressure and temperature, no organic solvents, and ease of promotion. Unfortunately, due to continuous collisions of materials during milling, the synthesized MOF has a low specific surface area (usually below 150 m2 g-1), making practical applications difficult. Therefore, there is an urgent need for green and environmentally friendly methods that use little or no organic solvents to convert PET waste into high specific surface area MOF.
On the other hand, porous MOF has become a research hotspot in new green energy generation technology—water evaporation power generation technology. Water evaporation power generation is a new technology that converts the latent heat of water into electrical energy by driving water flow through a power generation device during water evaporation. When the electrolyte is filled with charged wall porous materials, a double electric layer forms on the inner surface of the nanopores, affecting the migration of positive and negative ions in the pores, leading to different concentrations of positive and negative ions at both ends of the pores. The directional migration of charged ions generates current during the pressure gradient-driven flow of the solution. Although significant progress has been made in water evaporation power generation technology, building low-cost, high-efficiency water evaporation generators remains a daunting challenge.
Work Introduction
In recent work (Chemical Engineering Journal, with an impact factor of 13.4, a top journal in the Chinese Academy of Sciences), we achieved the upgraded recycling of waste PET into high surface area MIL-53Al using a trace solvent-assisted two-step ball milling method and prepared a high-performance MIL-53Al water evaporation generator.
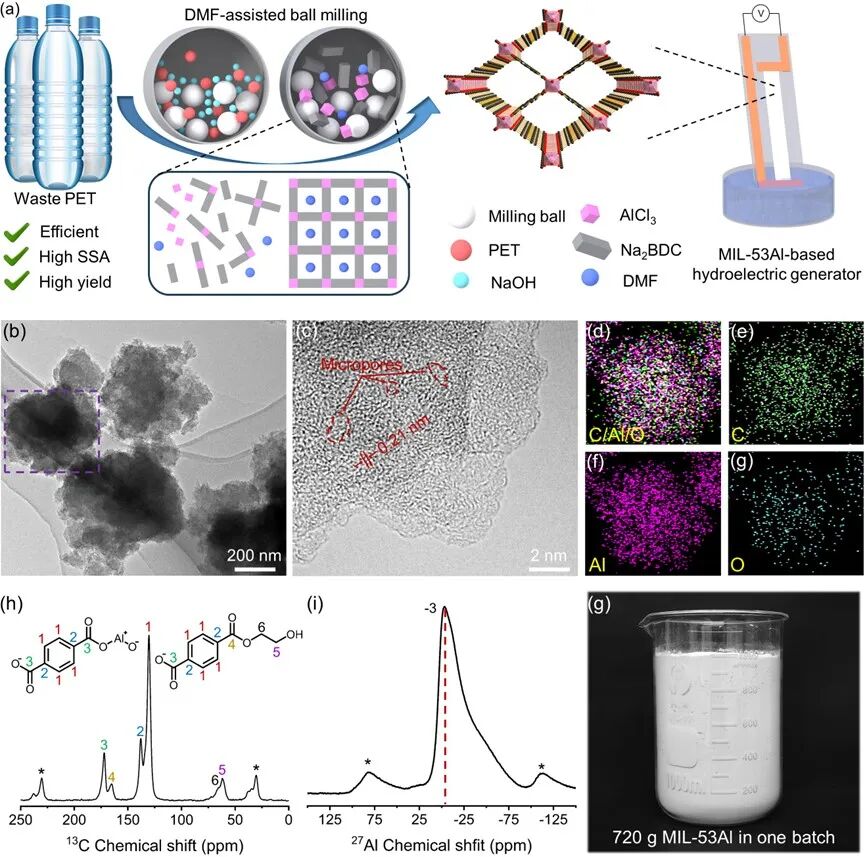
Figure 1 (a) Schematic diagram of the preparation of MIL-53Al for water evaporation power generation, (b and c) HR-TEM images of MIL-53Al, (d-g) EDX spectra of MIL-53Al, (h) 13C solid-state NMR spectrum of MIL-53Al; note: * indicates spinning sideband peaks, (i) 27Al solid-state NMR spectrum of MIL-53Al; note: * indicates spinning sideband peaks. (g) Photo of a batch of 720 grams of MIL-53Al.
Figure 1a shows the process of producing MIL-53Al from waste PET using a trace organic solvent-assisted two-step ball milling method. Waste PET is milled with NaOH to form Na2BDC; then AlCl3 and a small amount of DMF are added for milling to obtain MIL-53Al. The XRD pattern of the synthesized MIL-53Al shows characteristic diffraction peaks consistent with those of MIL-53Al synthesized by solvothermal methods, confirming the formation of the crystalline structure. As shown in the HR-TEM images (Figure 1b), uniform nanoparticles are deposited on the surface of MIL-53Al. MIL-53Al exhibits a porous structure with a lattice edge spacing of 0.21 nm, indicating the (011) plane of MIL-53Al (Figure 1c). The indistinct lattice edges suggest the presence of defects in the macroscopically long-range ordered growth, which may be caused by the harsh reaction environment during milling. The EDX spectrum results show that the distribution of C, Al, and O elements in MIL-53Al is uniform (Figures 1d-1g). The 13C solid-state NMR results show chemical shifts at 128, 140, and 160 ppm (Figure 1h) representing protonated aromatic carbons (1), non-protonated aromatic carbons (2), and ester carboxyl carbons (3). Notably, the chemical shift at 60 ppm indicates the presence of methylene carbons of ethylene glycol (5), which partially weakens the crystallinity of MIL-53Al and imparts functional groups to it. The 27Al NMR spectrum shows that the chemical shift at about -3 ppm corresponds to the octahedral [AlO4(OH)2] units in the framework. The broadening of signals from about -48 ppm to about -80 ppm represents the static average between open rhombic channels (dehydrated state) and closed rhombic channels (fully hydrated state). The peak broadening from about -3 ppm to about -90 ppm is caused by the residual water content in the pores.
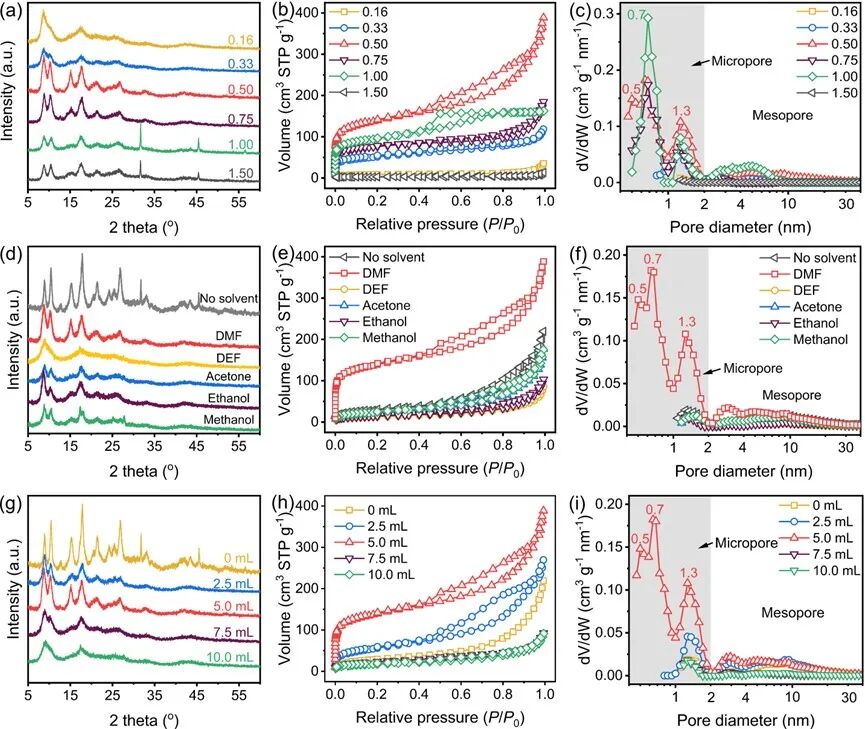
Figure2 XRD patterns, N2 adsorption-desorption curves, and simulated pore size distribution curves of MIL-53Al using (a-c) different AlCl3/Na2BDC molar ratios (0.16-1.5), (d-f) different organic solvents, and (g-i) different amounts of DMF (0-10.0 mL) in the preparation of MIL-53Al.
In the process of preparing MIL-53Al, we studied the effects of the molar ratio of AlCl3/Na2BDC, solvent type, and amount on the crystallization structure and pore structure of MIL-53Al. First, when the molar ratio of AlCl3/Na2BDC is below 0.50, some XRD diffraction peaks of MIL-53Al are missing, indicating that its crystal structure is incomplete (Figure 2a). When the molar ratio of AlCl3/Na2BDC exceeds 0.75, two new diffraction peaks appear at 2θ = 32° and 45°, which is due to excessive metal coordination centers leading to multiple coordination forms. Similarly, as the molar ratio of AlCl3/Na2BDC increases from 0.16 to 0.50, the specific surface area of MIL-53Al increases from 32 m² g-1 to 590 m² g-1 (Figure 2b), and more pores of size 0.5-2.0 nm are formed in MIL-53Al (Figure 2c). The specific surface area of MIL-53Al is lower than that of previously studied MIL-53Al due to pore collapse and framework deformation caused by strong mechanical shear forces during milling. When the molar ratio of AlCl3/Na2BDC increases from 0.50 to 1.50, the specific surface area decreases from 590 m² g-1 to 9 m² g-1. In contrast, when the molar ratio of AlCl3/Na2BDC is 0.50, adding other organic solvents such as DEF, ethanol, methanol, and acetone results in incomplete crystallization structure and fewer porous structures of the product MIL-53Al (Figures 2d-2f). Therefore, DMF plays a key role in the growth of MIL-53Al. Additionally, as the amount of DMF increases from 0 mL to 5 mL, the crystallization structure of MIL-53Al becomes more complete (Figure 2g), and the specific surface area of MIL-53Al increases from 104 m² g-1 to 590 m² g-1 (Figures 2h and 2i). Interestingly, when the amount of DMF exceeds 5 mL, the characteristic peaks of MIL-53Al decrease or even disappear, and the specific surface area of MIL-53Al decreases from 590 m² g-1 to 63 m² g-1, which may be due to excessive DMF weakening the mechanical forces during milling, leading to incomplete reactions.
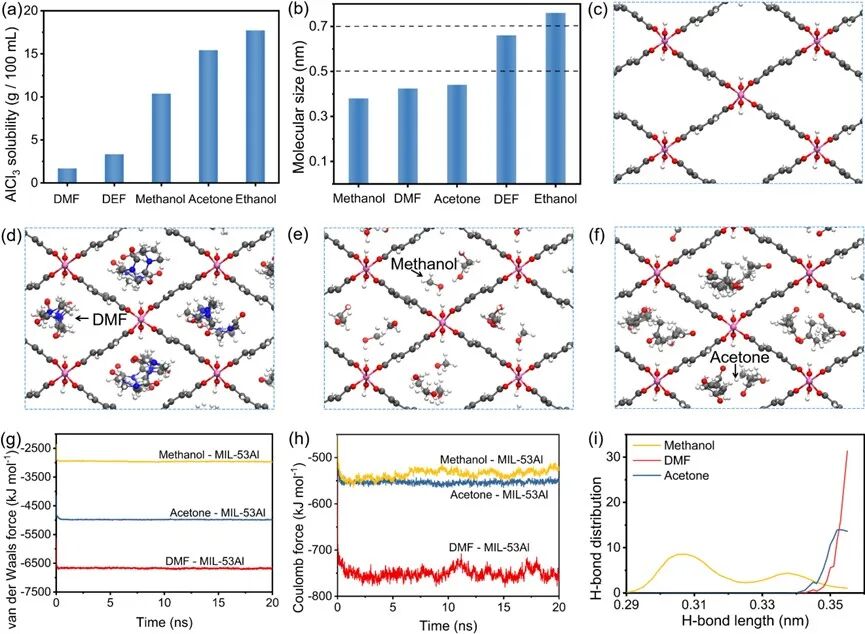
Figure3 (a) Solubility of AlCl3 in different solvents, (b) Molecular sizes of different solvents, (c) Framework structure diagram of MIL-53Al. The framework of MIL-53Al compared with (d) DMF, (e) methanol, or (f) acetone structure diagrams. Comparison of (g) van der Waals forces and (h) Coulomb forces between different solvent molecules and the framework of MIL-53Al, (i) Hydrogen bond length distribution diagram between different solvent molecules and the framework of MIL-53Al.
To investigate the specific role of DMF in the growth process of MIL-53Al, we first tested the solubility of AlCl3 and Na2BDC in different organic solvents (Figures 3a and 3b). AlCl3 has much lower solubility in DMF than in DEF, methanol, acetone, and ethanol, while Na2BDC has similar solubility in different solvents. Therefore, the solubility of AlCl3 and Na2BDC in DMF should not be a key factor in the growth process of MIL-53Al. Next, we compared the molecular sizes of different organic solvents. The molecular sizes of DEF and ethanol exceed the pore size of MIL-53Al, making them unsuitable as molecular templates for constructing stable frameworks of MIL-53Al. This explains the lower specific surface area of MIL-53Al when using DEF or ethanol. In contrast, methanol, DMF, and acetone can serve as molecular templates because their molecular sizes are smaller than the pore size of MIL-53Al. Specifically, during the growth of MIL-53Al, small-sized solvent molecules can fill the pore framework of MIL-53Al, maintaining an ordered framework structure even in a high mechanical strength environment, which is conducive to the growth of MIL-53Al with high specific surface area. In contrast, large-sized solvent molecules cannot enter the pore framework of MIL-53Al, leading to a lower specific surface area of MIL-53Al. Compared to methanol and acetone, the role of DMF is the most prominent, thus molecular dynamics (MD) simulations were applied to study the interactions between the framework of MIL-53Al and DMF/methanol/acetone. As expected, all three solvent molecules can enter the framework of MIL-53Al (Figures 3c-f). Figures 3g and 3h show the interactions between different solvent molecules and the framework of MIL-53Al. Compared to methanol and acetone, DMF exhibits the strongest van der Waals and Coulomb forces with the framework of MIL-53Al, indicating that DMF can better stabilize the framework of MIL-53Al during milling, thereby improving the crystallization structure and specific surface area. Furthermore, the hydrogen bond lengths formed between the three solvent molecules and the framework of MIL-53Al (Figure 3i) are longer than the conventional hydrogen bond lengths (generally 1.8-2.5 Å), indicating that during the growth of MIL-53Al, the hydrogen bonds between solvent molecules and the framework of MIL-53Al can be neglected.
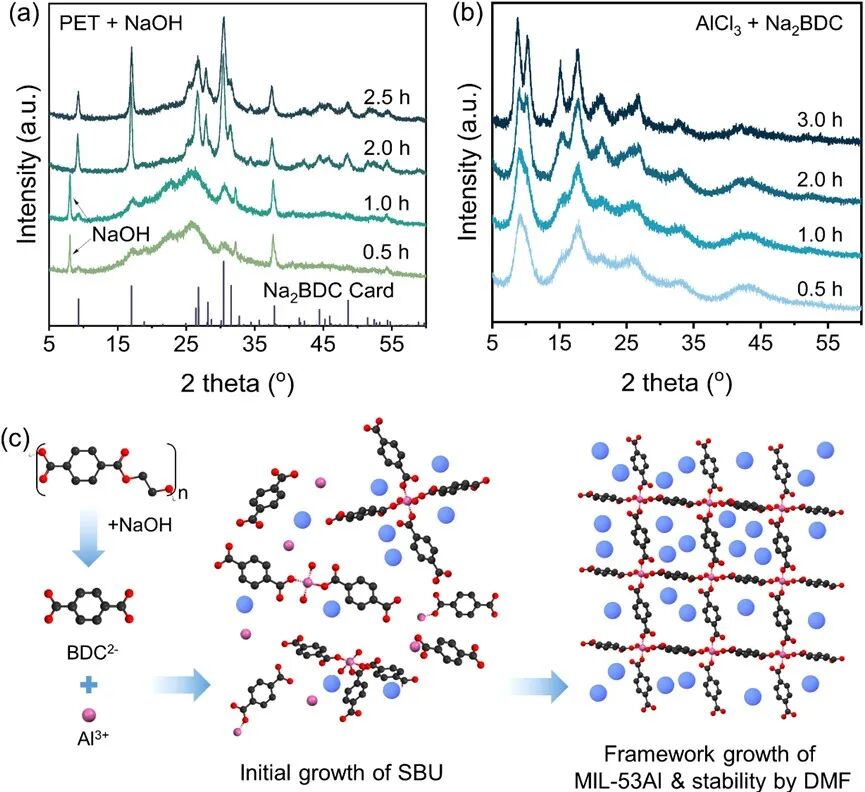
Figure4 (a) Intermediate degradation products of PET or (b) XRD spectra of intermediate products. (c) Growth mechanism of MIL-53Al.
To study the formation kinetics of MIL-53Al, we investigated the degradation of PET and the coordination of BDC2- with Al3+. As the milling time increases, the diffraction peak at 2θ = 7° belonging to NaOH weakens and disappears after 2 hours (Figure 4a). The diffraction peaks of Na2BDC appear at 2θ = 9°, 17°, 26°, and 32° after 0.5 hours and reach their strongest intensity after 2.5 hours. In the second ball milling, the characteristic peaks of MIL-53Al appear at 2θ = 9°, 12°, and 20°, becoming stronger with prolonged milling time (Figure 4b). Finally, the characteristic peaks of MIL-53Al reach their maximum intensity, while the characteristic peaks of Na2BDC completely disappear, proving that Al3+ and BDC2- rapidly coordinate to form MIL-53Al fragments, which gradually grow into long-range ordered structures. Based on the above findings, we propose the growth mechanism of MIL-53Al, which is achieved through the two-step ball milling of PET with the assistance of trace solvents (Figure 4c). First, PET is degraded into BDC2- through an alkaline hydrolysis process in the solid state. Subsequently, the coordination between BDC2- and Al³⁺ generates secondary structural units, which self-assemble to form the framework of MIL-53Al. In this process, DMF stabilizes the formation of the ordered framework of MIL-53Al, which is crucial for maintaining the crystalline framework and ultimately enhances the specific surface area of MIL-53Al. In summary, the templating and stabilizing effects of DMF not only improve the crystallinity of MIL-53Al but also increase the specific surface area, overcoming the inherent limitations of traditional solvent-free ball milling methods.
Using polydopamine (PDA ) as a binder to construct stable MIL-53Al water evaporation generators. As shown in Figure 5a, MIL-53Al is dispersed in a PDA solution. The mixture is then coated onto cotton fabric and fixed onto a PET sheet with conductive copper adhesive to create the MIL-53Al/PDA water evaporation generator (simplified as MPH). It can be seen that the MIL-53Al particles are wrapped and attached to the cotton fabric by PDA, forming micron-sized particles (Figure 5b). The open-circuit voltage and short-circuit current of the MPH device are approximately 270 mV and 14 μA (Figures 5c and 5d), significantly higher than those of the PDA device (70 mV and 4 μA) or the MIL-53Al device (110 mV and 6 μA). Therefore, the combination of PDA and MIL-53Al exhibits a synergistic effect in water evaporation power generation. The ζ potentials of MIL-53Al and PDA are 18 and 38 mV, respectively, allowing them to form an electronegative double layer membrane in seawater and exhibit similar ion selectivity. Furthermore, MIL-53Al has a porous structure, and the PDA particles fill the gaps in the MIL-53Al particles, thereby constructing ion channels throughout the generator. Therefore, the combination of the porous structure and ion channels ultimately enhances the power generation performance of the device. To further investigate the synergistic mechanism of water evaporation power generation in the MPH device, MD simulations were applied to study the migration of Na+ and Cl– in seawater within the MPH device. Figure 5 shows a schematic diagram of the simulation system, which includes MIL-53Al, PDA, Na+, Cl–, and H2O. The diffusion coefficients of Na+ and Cl– in the MIL-53Al, PDA, and MPH generators were calculated (Figure 5f). The results show that the difference in diffusion coefficients of Na+ and Cl– is the largest in the MPH, while the smallest in the PDA generator. The difference in diffusion coefficients of Na+ and Cl– has a significant impact on voltage generation. The difference in diffusion coefficients of Na+ and Cl– leads to different ion concentrations at the positive and negative electrodes of the MPH, ultimately generating voltage. Additionally, the radial distribution function (RDF) was used to analyze the interactions between Na+, Cl–, PDA, and MIL-53Al. The results show that compared to PDA in the MPH, the interactions between MIL-53Al and Na+ and Cl– are stronger, indicating that MIL-53Al plays an important role in water evaporation power generation. Within the ion channels, the transport speed of Cl– is faster than that of Na+, resulting in net charge accumulation that generates voltage (Figure 5h). Furthermore, we prepared an MPH generator with dimensions of 10 cm × 20 cm for outdoor water evaporation power generation experiments (Figures 5i and 5j). After 20000 seconds, the open-circuit voltage reached 300 mV, and the short-circuit current reached 15 μA (Figure 5k), indicating its potential for application in water evaporation power generation.
The above research results were published in the journal Chemical Engineering Journal under the title Trace solvents-assisted mechanochemistry of waste poly(ethylene terephthalate) into MIL-53Al for efficient hydroelectricity generation (impact factor of 13.4, top journal in the Chinese Academy of Sciences). The first author of the paper is Yan She, a master’s student from the School of Chemistry and Chemical Engineering at Huazhong University of Science and Technology, and the corresponding author is Researcher Jiang Gong from Huazhong University of Science and Technology. The co-authors include master’s students Weikang Han, Huajian Liu, Guixin Hu, and Xinyao Zhang, as well as doctoral students Huiyue Wang, Xueying Wen, and Lingling Feng. This research was supported by the National Natural Science Foundation and other funding sources.
Article Information
Yan She, Weikang Han, Huajian Liu, Guixin Hu, Huiyue Wang, Xueying Wen, Lijie Liu, Lingling Feng, Xinyao Zhang, Jiang Gong*. Trace solvents-assisted mechanochemistry of waste poly(ethylene terephthalate) into MIL-53Al for efficient hydroelectricity generation. Chemical Engineering Journal, 2025, 515, 163895
https://doi.org/10.1016/j.cej.2025.163895
First Author Introduction

Yan She, a master’s student from the School of Chemistry and Chemical Engineering at Huazhong University of Science and Technology, graduated with a bachelor’s degree from Huazhong University of Science and Technology. He has published a total of 8 SCI papers, including 2 as the first author, published in Chemical Engineering Journal and Nano Research; he has applied for 8 Chinese invention patents, including 1 as the second applicant. His research focuses on upgrading the recycling of waste PET into MOF materials and studying the properties of solar interface water evaporation and water evaporation power generation. He has received various honors, including the second-class academic scholarship from Huazhong University of Science and Technology, the first prize in the 15th Hubei Province College Student Chemistry (Chemical Engineering) Academic Innovation Achievement Report in 2022, the first prize in the 17th Hubei Province College Student Chemistry (Chemical Engineering) Academic Innovation Achievement Report in 2024, and the third prize in the finals of the 9th “Maker China” Hubei Province SME Innovation and Entrepreneurship Competition (6th place).