
【Citation Format】Li Mian, Tan Zhonglin, Wu Yue, et al. Research advances in glutamate and γ-aminobutyric acid homeostasis imbalance in major depressive disorder[J]. Chinese Journal of Nervous and Mental Diseases, 2023, 49(12): 758-764.
【Cite this article】LI Mian, TAN Zhonglin, WU Yue, et al. Research advances in glutamate and γ-aminobutyric acid homeostasis imbalance in major depressive disorder[J]. CHINESE JOURNAL OF NERVOUS AND MENTAL DISEASES, 2023, 49(12): 758-764.
DOI:10.3969/j.issn.1002-0152.2023.12.010
Research Advances in Glutamate and γ-Aminobutyric Acid Homeostasis Imbalance in Major Depressive Disorder
LI Mian TAN Zhonglin WU Yue LIANG Sugai
Affiliated Mental Health Centre, Hangzhou Seventh People’s Hospital, Zhejiang University School of Medicine
Abstract: Abnormal homeostasis of glutamate (Glu) and γ-aminobutyric acid (GABA) in the brain is one of the pathophysiological mechanisms of brain dysfunction in major depressive disorder (MDD). Neurotransmitters play an important role in maintaining chemical balance in the brain, and pharmacological and non-pharmacological therapies based on resetting excitation-inhibitory neurotransmitter system rebalancing are of interest. Studies based on magnetic resonance spectroscopy (MRS) have shown a homeostasis imbalance of Glu and GABA in the brain of MDD patients. Pharmacological therapies such as ketamine, selective serotonin reuptake inhibitors, and other novel receptor modulators and non-pharmacological therapies such as repetitive transcranial magnetic stimulation, electroconvulsive therapy, and physical exercise can target the regulation of neurotransmitter levels. Abnormal homeostasis of Glu and GABA provides theoretical support for revealing pathophysiologic mechanisms of MDD, exploring neurotransmitter biomarkers, guiding clinical practice, and facilitating personalized treatment.
Keywords:
Major depressive disorder; Glutamate; γ-aminobutyric acid; Homeostasis imbalance; Magnetic resonance spectroscopy; Targeted regulation; Treatment
Major depressive disorder (MDD) is a common clinical mental disorder with complex etiology and pathogenesis. Studies have found that the imbalance of brain glutamate (Glu) and γ-aminobutyric acid (GABA) homeostasis is closely related to functional abnormalities in brain regions, inducing the occurrence and development of MDD. Glu is an important excitatory neurotransmitter in the central nervous system, while GABA, as the main inhibitory neurotransmitter in the brain, fine-tunes and comprehensively controls excitatory transmission processes, making the regulation of Glu/GABA homeostasis rebalancing significant for the pathophysiological research and treatment of MDD.
1 Brain Glu and GABA Metabolic Cycle
The homeostasis of Glu/GABA in the brain is crucial for maintaining normal neuronal and synaptic function. The imbalance of these neurotransmitters in different brain regions is considered one of the pathophysiological mechanisms of MDD and a focal point in the mechanism of antidepressant action. The balance of Glu/GABA homeostasis in the central nervous system is primarily maintained by the glutamate-glutamine cycle between neurons and astrocytes. Extracellular Glu binds to excitatory amino acid transporters on the membrane and enters astrocytes, where it is converted into glutamine (Gln) by glutamine synthetase and transported to presynaptic nerve terminals. Gln is then converted back to Glu in the cytoplasm by glutaminase; extracellular Glu can also be directly transported to presynaptic cytoplasm via presynaptic membrane excitatory amino acid transporters, terminating the extracellular action of Glu. GABAergic neurons uptake Glu from the cycle, and through the action of glutamic acid decarboxylase (GAD), it is catalyzed into GABA and released into the synaptic cleft, where it is taken up by astrocytes and converted into Gln via the tricarboxylic acid cycle, ultimately becoming GABA through the continuous action of glutaminase and GAD. Abnormal Glu cycling in the brain is closely related to the pathophysiology of MDD. Studies have shown that in depressed mice, the metabolic cycle and activity of neurotransmitters dominated by glutamatergic and GABAergic neurons in the prefrontal cortex are reduced, leading to decreased conversion of Glu to Gln and GABA, resulting in the accumulation of extracellular Glu, causing excitotoxicity, damaging glial cells, and reducing synaptic connectivity.
2 Glu/GABA Neurotransmitter Homeostasis Imbalance
Research using brain functional imaging techniques in MDD patients and depressed animal models has found abnormal changes in Glu and GABA levels and activity in key brain regions such as the hippocampus, prefrontal cortex, anterior cingulate cortex (ACC), occipital cortex (OCC), dorsal striatum (caudate nucleus and putamen), and amygdala, leading to excitation-inhibition homeostasis. Studies have shown that chronic stress causes neuronal atrophy in the cortical-limbic brain regions of MDD patients, disrupting connectivity and function, and causing neurotransmitter dysregulation, which compromises the integrity of signaling pathways and induces depressive behavior. Research related to Glu/GABA neurotransmitter homeostasis imbalance in MDD patients is shown in Table 1.Table 1: Research on Glu/GABA neurotransmitter homeostasis imbalance in major depressive disorder based on 1H-MRS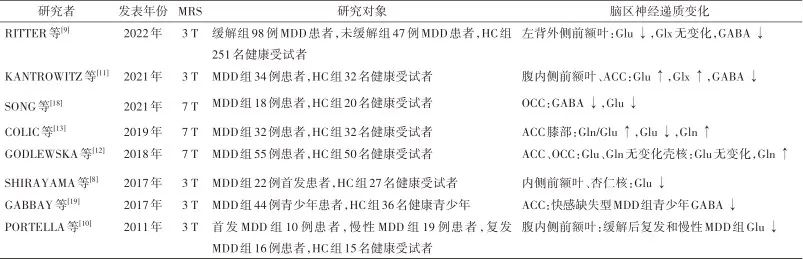
Note:1H-MRS, proton magnetic resonance spectroscopy; MDD, major depressive disorder; HC, healthy control; Glu, glutamate; Gln, glutamine; Glx, glutamate-related compounds; GABA, γ-aminobutyric acid; ACC, anterior cingulate cortex; OCC, occipital cortex.
2.1 Changes in Brain Glu and Its Metabolite Levels SHIRAYAMA et al.[8] found that in first-episode, medication-free MDD patients, Glu levels in the medial prefrontal cortex were significantly decreased, while Glu levels in the amygdala were reduced, and there were no changes in hippocampal Glu levels. The Glu levels in the medial prefrontal cortex and amygdala were significantly correlated with executive function, while hippocampal Glu levels were significantly correlated with memory function. RITTER et al.[9] found that compared to healthy controls, medication-free MDD patients in remission had lower Glu levels in the left dorsolateral prefrontal cortex. PORTELLA et al.[10] found that compared to first-episode MDD patients, those with recurrent and chronic MDD had lower Glu levels in the ventromedial prefrontal cortex, and the reduction in Glu levels was associated with more severe depressive symptoms and longer duration of illness. KANTROWITZ et al.[11] found that in medication-free MDD patients, Glu and glutamate-related compound (Glx) levels in the ventromedial prefrontal cortex and ACC increased after two weeks of no medication, and Glu and Glx levels were positively correlated with the severity of depressive symptoms. GODLEWSKA et al.[12] found that in medication-free MDD patients, there were no significant changes in Glu and Gln levels in the ACC and OCC based on 7 T-MRS, while Gln levels in the putamen were significantly elevated, with no changes in Glu levels. COLIC et al.[13] found that compared to healthy controls, MDD patients had an increased Gln/Glu ratio in the ACC knee, which was associated with the severity of depressive symptoms; the Gln/Glu ratio was significantly elevated in the medication-free group, while there was no statistical difference in the medication treatment group. This indicates that pharmacological treatment has a regulatory effect on the Gln/Glu ratio, and the levels of Glu and its metabolites in the brain of MDD patients are easily influenced by medication. The above research results indicate that MDD patients exhibit changes in Glu and its metabolite levels in the prefrontal cortex, ACC knee, OCC, amygdala, and putamen, but the research results are not entirely consistent. The possible reasons can be summarized as follows. First, the changes in Glu levels in the brain of MDD patients are influenced by multiple factors, not only related to abnormalities in Glu synthesis, metabolism, and reuptake processes but also closely related to the characteristics of the illness, severity of symptoms, treatment, and immune inflammation. Second, Gln levels are jointly influenced by Glu cycling and GABA levels, and the release of neurotransmitters in the synapse and Glu cycling is a highly dynamic process, making the interpretation of changes in Glu and Gln measurements at a single time point in MDD patients temporary and speculative. The metabolic pathways and mechanisms of neurotransmitters in the brain are complex, and the levels of Glu and its metabolites exhibit different changes in different brain regions, with variations in results even within the same brain region, which are related to the clinical subtypes of MDD, duration of illness, and medication use.2.2 Changes in Brain GABA Levels Multimodal brain imaging studies have shown that GABA levels are associated with functional connectivity in brain regions and reward circuit task activities, with GABA levels having a regulatory effect on the reward process. Studies have found that GABA levels in the cerebrospinal fluid and plasma of MDD patients are decreased, and postmortem brain tissue shows reduced expression of GAD67 in the prefrontal cortex. In chronic stress depression model mice, GABA, vesicular GABA transporter, and GAD65 are reduced, while levels of excitatory amino acid transporter 1 in the hippocampus are upregulated. SONG et al.[18] based on 7 T-MRS research showed that compared to healthy controls, depressed patients with visual perception disorders had significantly reduced GABA and Glu levels in the OCC, with the decrease in OCC GABA levels playing an important role in visual perception disorders in depressed patients. GABBAY et al.[19] found that in MDD patients with anhedonia, GABA levels in the ACC were decreased, while no significant decrease was observed in non-anhedonia patients. The GABA levels in MDD patients were significantly negatively correlated with the severity of anhedonia; moreover, compared to patients with a single depressive episode, those with recurrent depressive episodes had lower GABA levels in the ACC. Meta-analysis showed that GABA levels in the brain cortex, cerebrospinal fluid, and plasma of patients during depressive episodes were significantly reduced, while there was no significant decrease in GABA levels during remission or medication treatment. The decrease in GABA levels in MDD patients is related not only to the reduced density of GABAergic interneurons, decreased expression of GAD65/67, and increased excitotoxicity of Glu but also closely related to clinical subtypes of MDD and medication use. The clinical manifestations of MDD are highly heterogeneous, and the pathogenesis is related to the joint action of multiple brain regions. Future research should focus on the changes in Glu and GABA levels in different brain regions based on symptoms such as anhedonia and non-suicidal self-injury to further reveal the pathophysiological mechanisms of MDD and guide clinical practice.
3 Treatment and Interventions Related to Glu/GABA Homeostasis Imbalance
MDD is not only related to abnormal neurotransmitter levels in the brain but also to changes in the function of glutamatergic and GABAergic receptors. Long-term neurotransmitter imbalance leads to adaptive changes in receptor function, affecting the quantity and density of the receptors themselves and involving receptor signaling pathways. Targeted regulation of Glu and GABA levels in brain regions through pharmacological and non-pharmacological treatment methods may promote personalized treatment for MDD patients. Research on the regulation of Glu/GABA neurotransmitter rebalancing for the treatment of MDD is shown in Table 2.Table 2: Research on the regulation of Glu/GABA neurotransmitter rebalancing for the treatment of major depressive disorder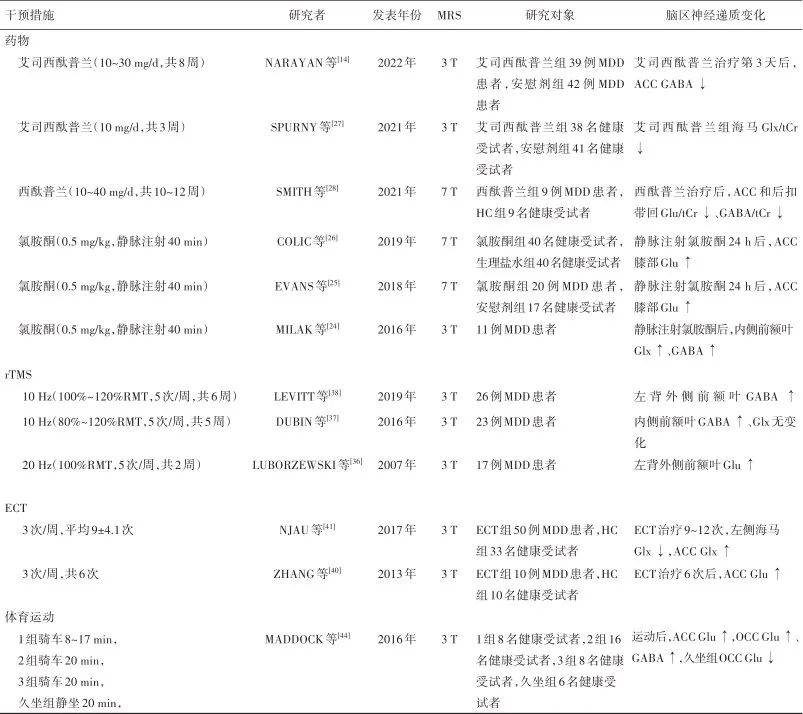
Note:MDD, major depressive disorder patients; HC, healthy control; MRS, magnetic resonance spectroscopy; rTMS, repetitive transcranial magnetic stimulation; RMT, resting motor threshold; ECT, electroconvulsive therapy; Glu/tCr, glutamate/total creatine; Gln, glutamine; Glx/tCr, glutamate-related compounds/total creatine; GABA/tCr, γ-aminobutyric acid/total creatine; ACC, anterior cingulate cortex; OCC, occipital cortex.
3.1 Ketamine Ketamine, a rapid-acting antidepressant, is an N-methyl-D-aspartate receptor (NMDAR) antagonist that can penetrate the blood-brain barrier, promote synaptic plasticity and local protein synthesis, and has rapid and lasting antidepressant effects, reducing suicidal ideation. Studies have shown that ketamine selectively blocks NMDAR on GABAergic interneurons, leading to reduced activation of inhibitory GABAergic receptors on pyramidal neurons in the prefrontal cortex, resulting in a disinhibition of Glu release. It also promotes the formation of new synapses by regulating downstream signaling pathways of synaptic plasticity. On the other hand, it blocks extrasynaptic NMDAR, inhibiting the phosphorylation of eukaryotic elongation factor 2 kinase, increasing the expression of brain-derived neurotrophic factor and synapse formation, and has a limiting effect on Glu excitotoxicity. The burst of Glu induced by ketamine is transient, and it can return to normal levels afterward, and the long-lasting synaptic plasticity it induces does not contradict the elevated Glu levels in the brain of MDD patients. Studies have found that after low-dose ketamine administration, Glu cycling in the medial prefrontal cortex of rats briefly increases, and the signaling of the glutamatergic system accelerates. MILAK et al.[24] found that in MDD patients, Glx and GABA levels in the medial prefrontal cortex significantly increased after intravenous ketamine injection. EVANS et al.[25] based on 7 T-MRS found that 24 hours after intravenous ketamine injection, Glu levels in the ACC knee of medication-free MDD patients showed an upward trend. Other studies have shown that Glu levels significantly increased in healthy subjects 24 hours after ketamine infusion. The above research results suggest that ketamine plays an important role in regulating Glu and GABA levels in brain regions such as the medial prefrontal cortex and ACC knee, often leading to increased levels of Glu and GABA.3.2 Selective Serotonin Reuptake Inhibitors SPURNY et al.[27] found that after healthy subjects took escitalopram (10 mg/d for 3 weeks), the Glx/total creatine (tCr) ratio in the hippocampus decreased, while the GABA/tCr ratio showed no significant change. Serotonergic drugs interact with glutamatergic neurotransmission, leading to downregulation of serotonergic receptor function in glutamatergic neurons and reduced Glu release. SMITH et al.[28] based on 7 T-MRS found that some elderly MDD patients showed decreased Glu/tCr and GABA/tCr ratios in the ACC and posterior cingulate cortex after treatment with citalopram (10-40 mg/d for 10-12 weeks), and the improvement of depressive symptoms was related to the decrease in the Glu/tCr ratio in the posterior cingulate cortex. NARAYAN et al.[14] found that in MDD patients, GABA levels in the ACC decreased on the third day of treatment with escitalopram (10-30 mg/d for 8 weeks), and there was no significant correlation between ACC GABA and Glx/GABA ratio levels and improvement of depressive symptoms after treatment. Current research indicates that both citalopram and escitalopram play a role in regulating Glu and GABA levels in the brain and maintaining the rebalancing of the excitation-inhibition neurotransmitter system.3.3 Other Novel Receptor Modulators Esketamine nasal spray is the enantiomer of ketamine, which has a high affinity for NMDAR as an antagonist and causes fewer side effects similar to ketamine, used for treating MDD patients with high suicide risk and strong suicidal ideation. Studies have shown that 4 hours after the first intranasal administration of esketamine, the severity of depression in MDD patients significantly decreased, and suicidal ideation improved. Auvelity (AXS-05, dextromethorphan combined with bupropion) is an oral rapid-acting NMDAR antagonist that can significantly improve depressive symptoms in MDD patients with minimal side effects. In 2022, the FDA officially approved Auvelity for the treatment of adult MDD. Additionally, brexanolone is an endogenous neuroactive steroid and a positive allosteric modulator of GABAA receptors. Brexanolone increases receptor expression and enhances inhibitory GABAergic signaling by modulating synaptic and extrasynaptic GABAA receptor subunits; it can also promote Glu release by inhibiting NMDAR, activating voltage-dependent calcium channels, leading to calcium influx, and ultimately activating the brain-derived neurotrophic factor/tyrosine kinase receptor B signaling pathway to produce antidepressant effects. The intravenous formulation of brexanolone (SAGE-547) was approved by the FDA in 2019 for the treatment of postpartum depression, while its oral formulation (Zuranolone, SAGE-217) is used for treating postpartum depression and adult MDD. Currently, there are few clinical application studies on glutamatergic and GABAergic receptor modulators based on 1H-MRS for changes in Glu and GABA levels in different brain regions, and future research can provide new evidence for the antidepressant mechanisms of receptor modulators based on Glu/GABA homeostasis rebalancing.3.4 Repetitive Transcranial Magnetic Stimulation Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive brain stimulation method that generates cortical currents through pulsed magnetic fields acting on the scalp, causing excitability changes in neurons and triggering functional connectivity effects between cortical and brain structures, affecting Glu/GABA metabolism in the brain and resetting the balance of excitation-inhibition neurotransmitter systems. LUBORZEWSKI et al.[36] treated MDD patients with 20 Hz-rTMS on the left dorsolateral prefrontal cortex (stimulation intensity 100% resting motor threshold, 5 times/week for 2 weeks) and found that after treatment, Glu levels in the dorsolateral prefrontal cortex increased, and overall depressive symptoms improved significantly, with Glu levels increasing positively correlated with stimulation intensity and clinical symptom improvement. DUBIN et al.[37] applied 10Hz-rTMS to MDD patients in the left dorsolateral prefrontal cortex (stimulation intensity 80%-120% resting motor threshold, 5 times/week for 5 weeks) and observed that GABA levels in the medial prefrontal cortex increased after treatment. LEVITT et al.[38] used 10 Hz-rTMS to treat MDD patients (stimulation intensity 100%-120% resting motor threshold, 5 times/week for 6 weeks) and found that GABA levels in the dorsolateral prefrontal cortex significantly increased in the effective treatment group, while no significant changes were observed in the ineffective treatment group, and the increase in GABA levels was not affected by the combination of GABAergic receptor agonists. A systematic review of studies based on 3 T-MRS showed that after rTMS treatment, Glu, Gln, and GABA levels increased in the left dorsolateral prefrontal cortex and ACC of MDD patients, and rTMS affects the glutamatergic and GABAergic systems in the prefrontal cortex, leading to enhanced downstream cellular signaling (through membrane depolarization and ion receptor modulation of synaptic transmission, while increasing the expression of vesicular glutamate transporter 1), thereby increasing the activity of glial cells in the prefrontal cortex to improve depressive symptoms. The above studies indicate that after rTMS treatment, the increase in Glu and GABA levels in the medial/lateral prefrontal cortex is associated with the improvement of depressive symptoms. Future research should expand the sample size to assess changes in Glu/GABA homeostasis in brain regions outside the prefrontal cortex and determine the relationship between Glu and GABA levels and different symptom dimensions such as anhedonia and anxiety.3.5 Electroconvulsive Therapy Electroconvulsive therapy (ECT) is a rapid and effective brain stimulation therapy for MDD. ZHANG et al.[40] observed that compared to healthy controls, medication-free MDD patients had lower Glu levels in the ACC, and after ECT treatment, the Glu levels in patients were not statistically different from healthy controls, with significantly increased ACC Glu levels in effective treatment responders compared to ineffective responders, suggesting that ACC Glu levels are related to the clinical efficacy of ECT. NJAU et al.[41] found that after ECT treatment, Glx levels in the left hippocampus of MDD patients decreased, while Glx levels in the ACC increased, and pointed out that chronic stress promotes increased Glu release in the hippocampus, leading to abnormal elevation of Glu levels, causing neuronal damage and loss of hippocampal cells, while ECT can block the excitotoxicity caused by high Glu levels in the hippocampus, restoring Glu/GABA homeostasis. The research results on changes in Glu and its metabolites in the brain regions of MDD patients after ECT treatment vary, which may be related to the characteristics of the illness and medication use. Future research should explore the correlation between changes in Glu and GABA levels and cognitive, emotional, and behavioral improvements in MDD patients based on different clinical subtypes.3.6 Physical Exercise Physical exercise can promote brain metabolism and improve cortical function, restoring hippocampal atrophy caused by chronic mental illness. Studies have shown that during exercise, GABAergic interneurons mediate the disinhibition of glutamatergic pyramidal neurons, significantly increasing the frequency of glutamatergic neurotransmission, causing a shift in brain cortical neural circuits towards excitation. MADDOCK et al.[44] found that after vigorous exercise, Glu levels in the ACC and OCC of healthy subjects increased, while GABA levels in the OCC significantly rose, indicating that physical exercise can promote the synthesis of Glu and GABA in the brain.
4 Conclusion and Outlook
Current research generally suggests that there is an imbalance in the central excitation-inhibition neurotransmitter system in the brains of MDD patients, typically characterized by elevated Glu levels and decreased GABA levels, leading to excitation dysregulation. This phenomenon helps to elucidate the important role of glutamatergic and GABAergic systems in the pathophysiological mechanisms of MDD. Research shows that ketamine, citalopram, and escitalopram play important roles in regulating Glu and GABA levels in brain regions such as the hippocampus, medial prefrontal cortex, ACC knee, and posterior cingulate cortex, maintaining the balance of excitation-inhibition homeostasis; novel receptor modulators targeting the glutamatergic and GABAergic systems significantly improve depressive symptoms. There are few clinical application studies based on 1H-MRS on changes in Glu and GABA levels in different brain regions, and future research should increase sample sizes to improve the reproducibility of studies and discover rapid and effective antidepressant treatment methods. Most studies show that after rTMS, ECT, and physical exercise treatment, Glu and GABA levels increase in brain regions such as the prefrontal cortex, ACC, and OCC, which has a certain effect on regulating the imbalance of excitation-inhibition neurotransmitter homeostasis in the brain. Currently, research exploring changes in Glu and GABA levels in different brain regions of MDD patients based on 1H-MRS after pharmacological and non-pharmacological treatments shows varying results, which may be due to individual clinical differences and the complex overlapping mechanisms of treatment effects, presenting challenges in measuring neurotransmitter levels, selecting optimal treatment plans, and conducting clinical trials. Furthermore, 1H-MRS cannot finely distinguish between intracellular and extracellular neurotransmitter levels, and research results are easily influenced by subjects, data collection, and analysis methods. Future research can combine 1H-MRS with multimodal brain imaging techniques to dynamically measure changes in Glu and GABA levels in different brain regions over time under different clinical subtypes of MDD and treatment interventions, potentially providing a more complete picture of the metabolic activity of neurotransmitters in the brain of MDD patients and their brain function.
1. FOGAÇA M V, DUMAN R S. Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions[J]. Front Cell Neurosci, 2019, 13: 87.
2. MARWAHA S, PALMER E, SUPPES T, et al. Novel and emerging treatments for major depression[J]. Lancet, 2023, 401(10371): 141-153.
3. SANACORA G, ZARATE C A, KRYSTAL J H, et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders[J]. Nat Rev Drug Discov, 2008, 7(5): 426-437.
4. SARAWAGI A, SONI N D, PATEL A B. Glutamate and GABA Homeostasis and Neurometabolism in Major Depressive Disorder[J]. Front Psychiatry, 2021, 12: 637863.
5. VEERAIAH P, NORONHA J M, MAITRA S, et al. Dysfunctional glutamatergic and gamma-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression[J]. Biol Psychiatry, 2014, 76(3): 231-238.
6. LENER M S, NICIU M J, BALLARD E D, et al. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine[J]. Biol Psychiatry, 2017, 81(10): 886-897.
7. DUMAN R S, SANACORA G, KRYSTAL J H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments[J]. Neuron, 2019, 102(1): 75-90.
8. SHIRAYAMA Y, TAKAHASHI M, OSONE F, et al. Myo-inositol, Glutamate, and Glutamine in the Prefrontal Cortex, Hippocampus, and Amygdala in Major Depression[J]. Biol Psychiatry Cogn Neurosci Neuroimaging, 2017, 2(2): 196-204.
9. RITTER C, BUCHMANN A, MÜLLER S T, et al. Evaluation of Prefrontal γ-Aminobutyric Acid and Glutamate Levels in Individuals with Major Depressive Disorder Using Proton Magnetic Resonance Spectroscopy[J]. JAMA Psychiatry, 2022, 79(12): 1209.
10. PORTELLA M J, DE DIEGO-ADELIÑO J, GÓMEZ-ANSÓN B, et al. Ventromedial prefrontal spectroscopic abnormalities over the course of depression: A comparison among first episode, remitted recurrent and chronic patients[J]. J Psychiatr Res, 2011, 45(4): 427-434.
11. KANTROWITZ J T, DONG Z, MILAK M S, et al. Ventromedial prefrontal cortex/anterior cingulate cortex Glx, glutamate, and GABA levels in medication-free major depressive disorder[J]. Transl Psychiatry, 2021, 11(1): 419.
12. GODLEWSKA B R, MASAKI C, SHARPLEY A L, et al. Brain glutamate in medication-free depressed patients: a proton MRS study at 7 Tesla[J]. Psychol Med, 2018, 48(10): 1731-1737.
13. COLIC L, VON DURING F, DENZEL D, et al. Rostral Anterior Cingulate Glutamine/Glutamate Disbalance in Major Depressive Disorder Depends on Symptom Severity[J]. Biol Psychiatry Cogn Neurosci Neuroimaging, 2019, 4(12): 1049-1058.
14. NARAYAN G A, HILL K R, WENGLER K, et al. Does the change in glutamate to GABA ratio correlate with change in depression severity? A randomized, double-blind clinical trial[J]. Mol Psychiatry, 2022, 27(9): 3833-3841.
15. DUNCAN N W, WIEBKING C, NORTHOFF G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans—A review of multimodal imaging studies[J]. Neurosci Biobehav Rev, 2014, 47: 36-52.
16. KAROLEWICZ B, MACIAG D, O’DWYER G, et al. Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression[J]. Int J Neuropsychopharmacol, 2010, 13(4): 411.
17. GARCIA-GARCIA A L, ELIZALDE N, MATROV D, et al. Increased Vulnerability to Depressive-Like Behavior of Mice with Decreased Expression of VGLUT1[J]. Biol Psychiatry, 2009, 66(3): 275-282.
18. SONG X M, HU X W, LI Z, et al. Reduction of higher-order occipital GABA and impaired visual perception in acute major depressive disorder[J]. Mol Psychiatry, 2021, 26(11): 6747-6755.
19. GABBAY V, BRADLEY K A, MAO X, et al. Anterior cingulate cortex γ-aminobutyric acid deficits in youth with depression[J]. Transl Psychiatry, 2017, 7(8): e1216.
20. SCHÜR R R, DRAISMA L W R, WIJNEN J P, et al. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of 1H-MRS studies[J]. Hum Brain Mapp, 2016, 37(9): 3337-3352.
21. ROMEO B, CHOUCHA W, FOSSATI P, et al. Meta-analysis of central and peripheral γ-aminobutyric acid levels in patients with unipolar and bipolar depression[J]. J Psychiatry Neurosci, 2018, 43(1): 58-66.
22. MURROUGH J W, ABDALLAH C G, MATHEW S J. Targeting glutamate signalling in depression: progress and prospects[J]. Nat Rev Drug Discov, 2017, 16(7): 472-486.
23. CHOWDHURY G M, ZHANG J, THOMAS M, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects[J]. Mol Psychiatry, 2017, 22(1): 120-126.
24. MILAK M S, PROPER C J, MULHERN S T, et al. A pilot in vivo proton magnetic resonance spectroscopy study of amino acid neurotransmitter response to ketamine treatment of major depressive disorder[J]. Mol Psychiatry, 2016, 21(3): 320-327.
25. EVANS J W, LALLY N, AN L, et al. 7T 1H-MRS in major depressive disorder: a Ketamine Treatment Study[J]. Neuropsychopharmacology, 2018, 43(9): 1908-1914.
26. COLIC L, MCDONNELL C, LI M, et al. Neuronal glutamatergic changes and peripheral markers of cytoskeleton dynamics change synchronically 24 h after sub-anaesthetic dose of ketamine in healthy subjects[J]. Behav Brain Res, 2019, 359: 312-319.
27. SPURNY B, VANICEK T, SEIGER R, et al. Effects of SSRI treatment on GABA and glutamate levels in an associative relearning paradigm[J]. Neuroimage, 2021, 232: 117913.
28. SMITH G S, OELTZSCHNER G, GOULD N, et al. Neurotransmitters and Neurometabolites in Late-Life Depression: A Preliminary Magnetic Resonance Spectroscopy Study at 7T[J]. J Affect Disord, 2022, 279: 417-425.
29. WILKINSON S T, SANACORA G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems[J]. Drug Discov Today, 2019, 24(2): 606-615.
30. CANUSO C M, SINGH J B, FEDGCHIN M, et al. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study[J]. Am J Psychiatry, 2018, 175(7): 620-630.
31. TABUTEAU H, JONES A, ANDERSON A, et al. Effect of AXS-05 (Dextromethorphan-Bupropion) in Major Depressive Disorder: A Randomized Double-Blind Controlled Trial[J]. Am J Psychiatry, 2022, 179(7): 490-499.
32. CHEN S, GAO L, LI X, et al. Allopregnanolone in mood disorders: Mechanism and therapeutic development[J]. Pharmacol Res, 2021, 169: 105682.
33. MELTZER-BRODY S, COLQUHOUN H, RIESENBERG R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials[J]. Lancet, 2018, 392(10152): 1058-1070.
34. CLAYTON A H, LASSER R, PARIKH S V, et al. Zuranolone for the Treatment of Adults with Major Depressive Disorder: A Randomized, Placebo‐Controlled Phase 3 Trial[J]. Am J Psychiatry, 2023, 180(9): 676-684.
35. FITZGERALD P B, FOUNTAIN S, DASKALAKIS Z J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition[J]. Clin Neurophysiol, 2006, 117(12): 2584-2596.
36. LUBORZEWSKI A, SCHUBERT F, SEIFERT F, et al. Metabolic alterations in the dorsolateral prefrontal cortex after treatment with high-frequency repetitive transcranial magnetic stimulation in patients with unipolar major depression[J]. J Psychiatr Res, 2007, 41(7): 606-615.
37. DUBIN M J, MAO X, BANERJEE S, et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy[J]. J Psychiatry Neurosci, 2016, 41(3): E37-E45.
38. LEVITT J G, KALENDER G, O’NEILL J, et al. Dorsolateral prefrontal γ-aminobutyric acid in patients with treatment-resistant depression after transcranial magnetic stimulation measured with magnetic resonance spectroscopy[J]. J Psychiatry Neurosci, 2019, 44(6): 386-394.
39. GONSALVES M A, WHITE T L, BARREDO J, et al. Repetitive Transcranial Magnetic Stimulation-Associated Changes in Neocortical Metabolites in Major Depression: A Systematic Review[J]. Neuroimage Clin, 2022, 35: 103049.
40. ZHANG J, NARR K L, WOODS R P, et al. Glutamate normalization with ECT treatment response in major depression[J]. Mol Psychiatry, 2013, 18(3): 268-270.
41. NJAU S, JOSHI S H, ESPINOZA R, et al. Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression[J]. J Psychiatry Neurosci, 2017, 42(1): 6-16.
42. BASKERVILLE R, MCGRATH T, CASTELL L. The effects of physical activity on glutamate neurotransmission in neuropsychiatric disorders[J]. Front Sports Act Living, 2023, 5: 1147384.
43. FU Y, TUCCIARONE J M, ESPINOSA J S, et al. A Cortical Circuit for Gain Control by Behavioral State[J]. Cell, 2014, 156(6): 1139-1152.
44. MADDOCK R J, CASAZZA G A, FERNANDEZ D H, et al. Acute Modulation of Cortical Glutamate and GABA Content by Physical Activity[J]. J Neurosci, 2016, 36(8): 2449-2457.