【Research Background】
Lithium-sulfur (Li-S) batteries are considered one of the most promising new secondary battery systems due to their high theoretical energy density, low cost, and environmental friendliness. However, their practical application is limited by challenges such as the shuttling effect caused by soluble polysulfides (LiPSs) and the kinetic hysteresis of the sulfur reduction reaction (SRR). Introducing catalytic strategies into the design of sulfur host materials has recently been proposed as an effective method to address these issues. Among these, single-atom catalysts (SACs) have gained favor among researchers due to their advantages of both homogeneous and heterogeneous catalysts. However, the preparation strategies for SACs typically rely on high-temperature pyrolysis, which not only increases energy consumption but also leads to uncontrollable random changes in the coordination environment of SACs during high-temperature pyrolysis, making rational design difficult.
Moreover, most reported SACs feature non-polar porphyrin-like coordination structures, which do not maximize the catalytic activity of the metal sites within the SACs. Therefore, how to utilize a more economical strategy to prepare SACs and enhance their catalytic activity through rational design of their coordination environment remains a significant challenge.
【Work Overview】
Recently, Professor Xiongwen Lou from City University of Hong Kong, along with Professors Zhiming Liu and Huifang Li from Qingdao University of Science and Technology, innovatively proposed a method based on Lewis acid-base theory. They designed a unique organic coordination environment with stronger electron-accepting ability to induce cobalt single-atom catalysts into an electron-deficient state, thereby enhancing their adsorption and catalytic conversion capabilities for LiPSs. Experimental results indicate that the electron-deficient Co SACs prepared using this strategy exhibit a stronger chemical affinity for LiPSs and effectively promote the conversion reactions among different sulfur species. This article, titled “Electron-deficient Cobalt Centers Realized by Rational p-π Conjugation Regulation for High-Performance Li-S Batteries,” was published in the prestigious journal Angewandte Chemie International Edition.
Doctoral student Mai Li from Qingdao University of Science and Technology is the first author of this paper.
【Content Description】
As the size of metal active sites decreases to the atomic level, their sensitivity to the coordination environment reaches its maximum, making the interaction between the metal center and the surrounding coordination environment one of the key factors determining catalytic performance. The CNT@f-CoNC designed in this study, due to its unique organic coordination environment formed through the Schiff base reaction, can effectively promote electron transfer from the cobalt sites to the surrounding coordination environment compared to the commonly reported porphyrin-like coordination environments. This induces the formation of electron-deficient Co SACs, which can achieve effective adsorption of LiPSs based on Lewis acid-base interactions and reduce the energy barrier of the SRR.
During the synthesis of CNT@f-CoNC, formamide (FA) molecules form C=N bonds through the Schiff base reaction, constructing a unique organic coordination environment without the need for pyrolysis treatment, and forming a stable composite structure with carbon nanotubes (CNTs) through π-π interactions on the surface. To further explore the differences between this unique organic coordination environment and the traditionally pyrolyzed porphyrin-like coordination environment, particularly their effects on the electronic structure of cobalt sites and the adsorption and catalytic performance of LiPSs, we further pyrolyzed CNT@f-CoNC to prepare cobalt single-atom catalysts (CNT@CoSA) with porphyrin-like structures.
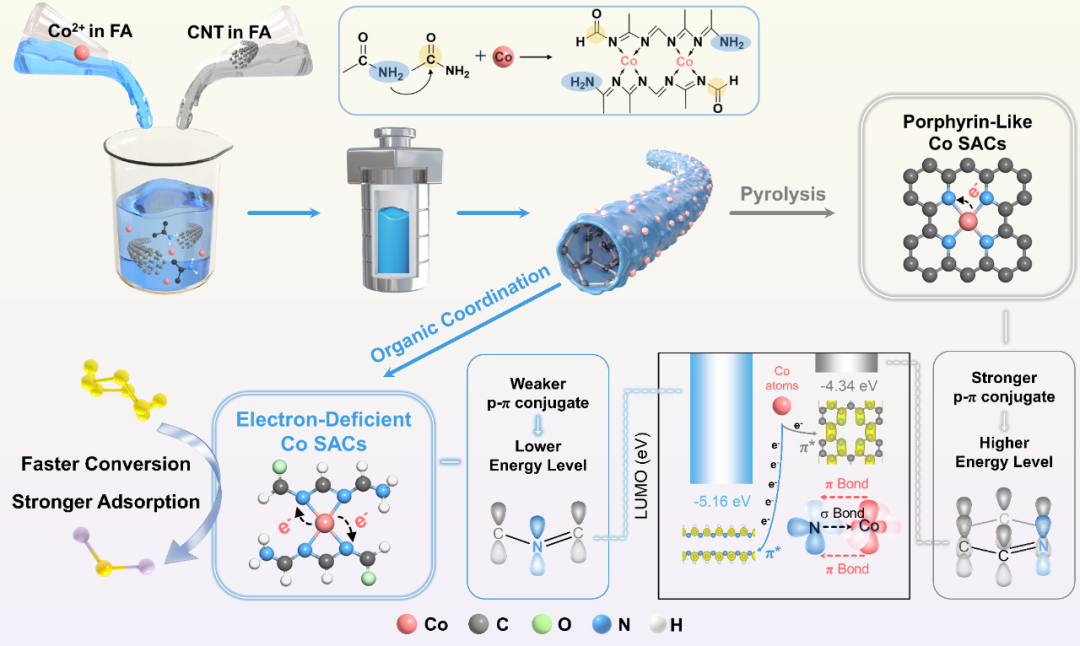
Figure 1. Schematic diagram of the synthesis of CNT@f-CoNC and CNT@CoSA with different coordination environments and different electronic states of Co SACs.
Theoretical calculations predict that the organic coordination environment constructed by formamide through the Schiff base reaction is more favorable for electron transfer at the cobalt sites compared to the commonly reported porphyrin-like coordination environments, thus facilitating the formation of electron-deficient Co SACs. Based on the above theoretical predictions, CNT@f-CoNC with electron-deficient Co SACs was successfully constructed using a one-step hydrothermal method. Furthermore, the electronic structures of CNT@f-CoNC and CNT@CoSA were analyzed in depth using X-ray photoelectron spectroscopy (XPS) and X-ray absorption fine structure (XAFS). The XPS results indicate that there is stronger electron transfer between the Co sites and N sites in CNT@f-CoNC, and the XANES spectra also show differences in the valence states of Co in CNT@f-CoNC and CNT@CoSA. This confirms the successful synthesis of electron-deficient Co SACs in CNT@f-CoNC. Transmission electron microscopy (TEM) and high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) observations reveal a uniformly coated ultrathin layer around the lattice of CNTs, confirming that the polymer derived from formamide achieves uniform coating on the CNT surface, and cobalt is observed to exist in an atomically dispersed form.
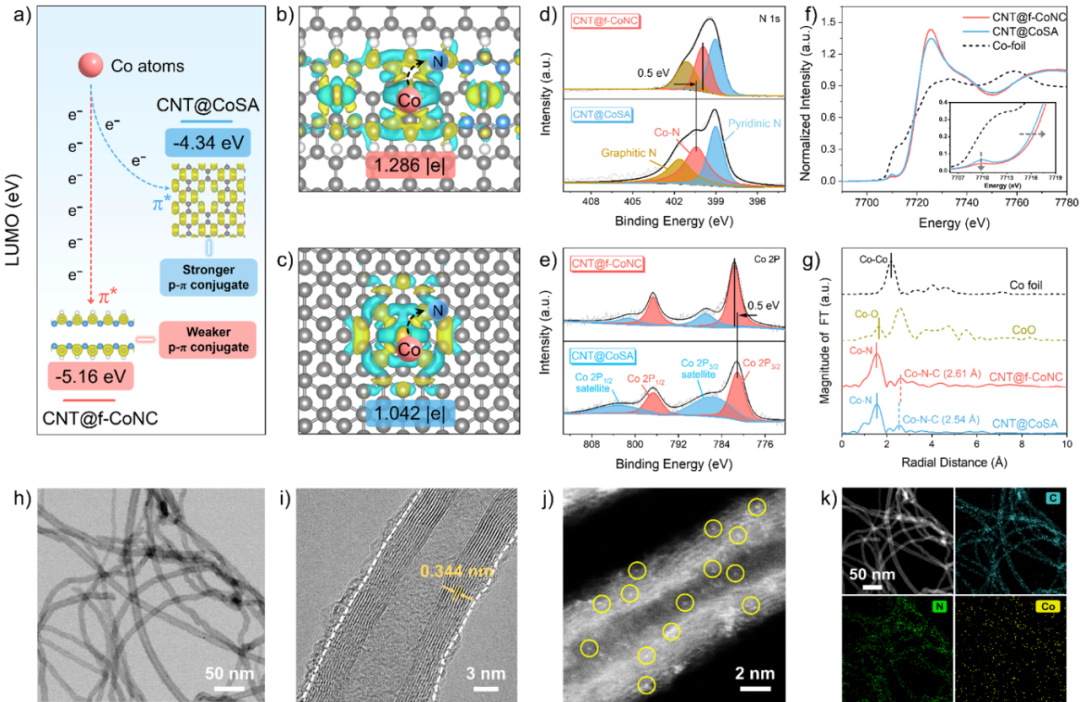
Figure 2. (a) LUMO energy levels and charge transfer schematic of CNT@f-CoNC and CNT@CoSA. (b,c) Top views of the atomic structures and differential charge of both. (d,e) XPS spectra analysis of N 1s and Co 2p for both. (f,g) Co K-edge XANES spectra and Fourier transform EXAFS spectra. (h-j) TEM, HRTEM, and HAADF-STEM images of CNT@f-CoNC. (k) Corresponding elemental mapping images.
We conducted detailed investigations into the ability of different electronic states of Co SACs to suppress the shuttling effect and accelerate the kinetics of sulfur species conversion through cyclic voltammetry (CV) curves, Tafel slopes, relative activation energies, shuttling currents, symmetric cells, and lithium sulfide deposition tests. Among them, CNT@f-CoNC exhibited a smaller shuttling current in the shuttling current test, indicating its effectiveness in suppressing the shuttling effect. Additionally, a series of experimental results from symmetric cell tests and lithium sulfide deposition tests demonstrate that CNT@f-CoNC has better catalytic effects on polysulfide conversion and can induce uniform deposition of lithium sulfide.
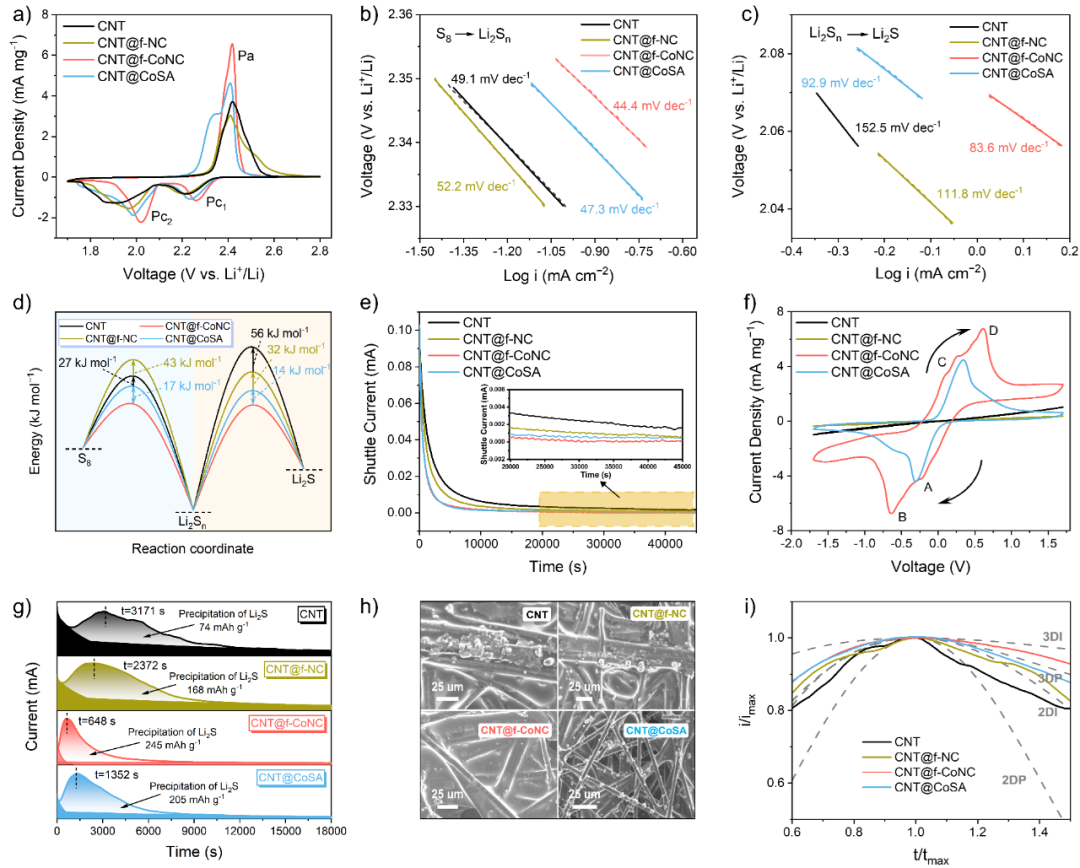
Figure 3. (a) Comparison of CV curves of each electrode. (b,c) Tafel slope analysis of the S8→Li2Sn and Li2Sn→Li2S reactions. (d) Comparison of reaction activation energies. (e) Shuttling current test results. (f) CV curves of the symmetric cell. (g) Lithium sulfide deposition test. (h) SEM images of the electrode surface after lithium sulfide deposition. (i) Dimensionless current-time transient curves.
Due to the excellent adsorption and catalytic capabilities of CNT@f-CoNC for polysulfides, the lithium-sulfur batteries assembled using CNT@f-CoNC as the sulfur host material exhibited significantly improved electrochemical performance. Notably, during over 300 cycles, a capacity retention rate of 90% was achieved. Furthermore, under harsh testing conditions with a high sulfur loading of 6.9 mg cm−2 and a low E/S ratio of 4.0 μL mg−1, the lithium-sulfur batteries based on CNT@f-CoNC still achieved a specific capacity of 7.7 mAh cm−2. Additionally, the assembled 1.6 Ah pouch battery demonstrated a mass energy density of 422 Wh kg−1, showcasing its potential for practical applications.
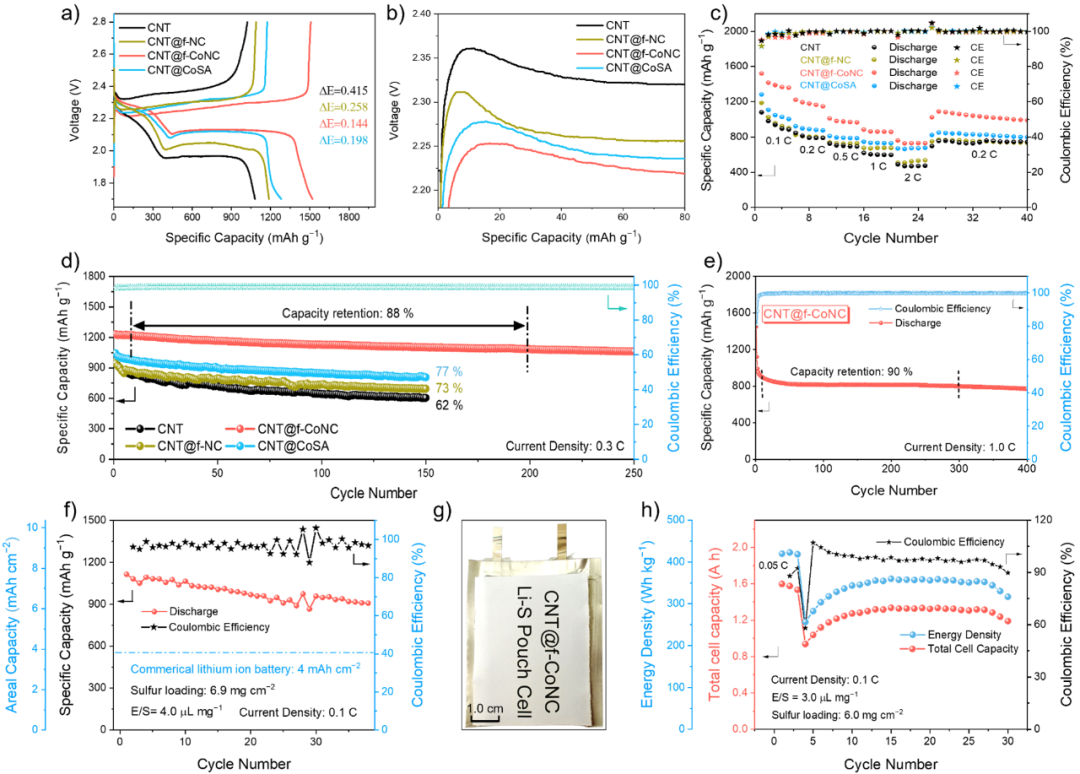
Figure 4. (a) First cycle charge-discharge curves. (b) Comparison of overpotential during the charging process. (c) Rate performance tests. (d) Cycle performance tests at 0.3 C. (e) Cycle performance tests at 1.0 C. (f) Performance tests under high sulfur loading and lean electrolyte conditions. (g) Optical photos of the pouch battery. (h) Performance tests of the pouch battery.
To gain a deeper understanding of the influence mechanism of electron-deficient Co SACs on the kinetics of LiPSs conversion reactions at the molecular scale, the authors conducted systematic density functional theory (DFT) calculations. The results indicate that the electron-deficient Co SACs in CNT@f-CoNC, due to their Lewis acid characteristics, exhibit stronger binding affinity for LiPSs, which possess Lewis base characteristics. Furthermore, due to the stronger intermolecular interactions between CNT@f-CoNC and LiPSs, the average S-S bond of the LiPSs molecules adsorbed on the CNT@f-CoNC surface is significantly elongated. This elongation of the S-S bond indicates a weakening of bond strength, which is more favorable for the conversion reactions among different sulfur species. Additionally, calculations regarding Gibbs free energy demonstrate that CNT@f-CoNC can effectively lower the reaction energy barrier of the rate-limiting step in the SRR, further promoting the conversion reactions of LiPSs.
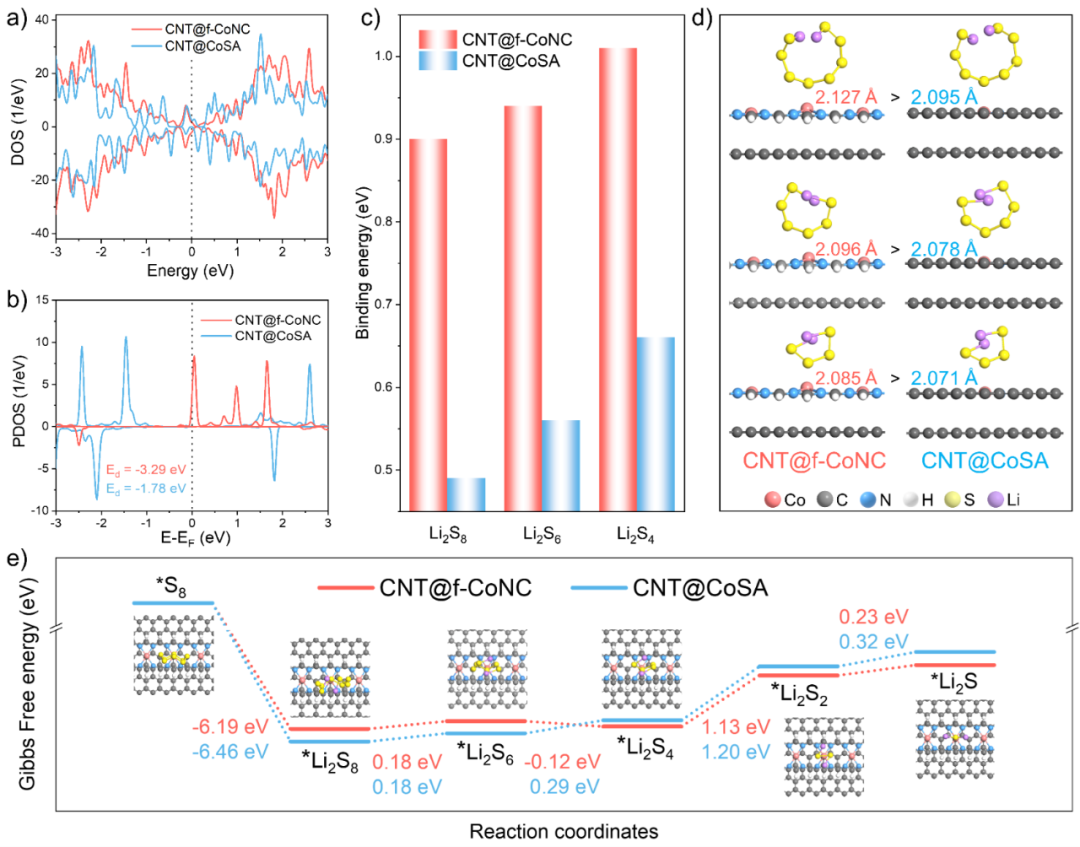
Figure 5. (a) Comparison of the density of states (DOS) of CNT@f-CoNC and CNT@CoSA. (b) Projected density of states (PDOS) of the Co-d orbitals and the d-band center positions for both. (c) Adsorption energies of both for LiPSs. (d) S-S bond stretching effect. (e) Changes in Gibbs free energy for the sulfur reduction reaction.
Core Conclusion
This work is inspired by the Lewis acid-base interaction mechanism between electron-deficient Co SACs and LiPSs, as well as the high sensitivity of SACs to their coordination environment. By designing a unique organic coordination environment, we successfully constructed electron-deficient Co SACs. Compared to the traditionally reported porphyrin structures, the uniquely constructed organic coordination environment in this work exhibits a lower LUMO energy level due to weaker p-π conjugation strength, resulting in stronger electron-accepting ability, which renders the Co sites in an electron-deficient state. The electron-deficient Co SACs, due to their Lewis acid characteristics, can enhance their adsorption and catalytic activity towards LiPSs (which possess Lewis base characteristics). Experimental results indicate that the pouch batteries assembled using CNT@f-CoNC can achieve a specific capacity of 7.7 mAh cm−2 under high sulfur loading and lean electrolyte conditions. Furthermore, the assembly of a 1.6 Ah pouch battery demonstrates the practical potential of this material. This work reveals the significant impact of the interaction between metal sites and their coordination environment on the catalytic activity of SACs, providing new insights for further enhancing the chemical adsorption and catalytic activity of SACs towards LiPSs.

【References】
Mai Li, Hui Liu, Huifang Li*, Deyan Luan, Zhiming Liu*, Xiong Wen (David) Lou*.
Electron-deficient Cobalt Centers Realized by Rational p-π Conjugation Regulation for High-Performance Li-S Batteries; DOI: 10.1002/anie.202503174.
【Author Profiles】
Professor Xiongwen Lou: Academician of the Academy of Science and Engineering of Singapore, currently a chair professor in the Department of Chemistry at City University of Hong Kong. He has been selected as a Clarivate Analytics (formerly Thomson Reuters) Highly Cited Researcher for 11 consecutive years from 2014 to 2024. His main research direction is the design and synthesis of nanostructured materials for energy and environmental-related fields. Professor Lou focuses on research in new energy materials and devices, achieving outstanding research results. In 2023, he received the 36th Khwarizmi International Award from the Iranian Ministry of Science and Technology, and in 2017, he was awarded the Readers’ Choice Lectureship Award by the Royal Society of Chemistry for the journal Energy & Environmental Science. He was elected a Fellow of the Royal Society of Chemistry (FRSC) in 2017, received the World Cultural Council (WCC) special recognition award in 2013, and was recognized as an Asian Rising Star at the 15th Asian Chemical Congress in 2013. He also received the Young Scientist Award from the Singapore National Academy of Sciences in 2012. In 2015, he was selected as a reviewer for the Singapore National Research Foundation (NRF) Investigatorship. Professor Lou also serves as the executive editor for Science Advances and is on the editorial boards of journals such as Chemical Communications, Chem, Chemical Science, Nano Letters, and Small Methods. He has published over 410 papers in top international journals, including Science, Nat. Energy, Sci. Adv., Angew. Chem. Int. Ed., Adv. Mater., J. Am. Chem. Soc., Nat. Comm., Chem, Joule, Energy Environ. Sci., and Adv. Energy Mater., with over 147,150 citations (Google Scholar) and an H-index of 227.
Professor Zhiming Liu: Professor at the School of Mechanical and Electrical Engineering, Qingdao University of Science and Technology, PhD supervisor, and a young expert of the Taishan Scholars program in Shandong Province. He graduated from Hanyang University in South Korea in August 2018 with a PhD. That same year, he joined the School of Mechanical and Electrical Engineering at Qingdao University of Science and Technology as a three-tier talent, focusing on teaching and research, and established an advanced energy storage technology research team (https://aest.qust.edu.cn/index.htm). The team was approved as a new battery storage materials and technology research innovation team by the Shandong Provincial Department of Education in 2021. His research focuses on the design and development of functional nanomaterials and their applications in energy storage and conversion technologies, such as lithium-sulfur batteries, metal-air batteries, and electrocatalysis. He has published over 60 articles as the first author or corresponding author in well-known international academic journals in the fields of materials, energy, and electrochemistry, including Angew. Chem. Int. Ed., Energy Environ. Sci., Adv. Energy Mater., Adv. Funct. Mater., ACS Catal., Energy Storage Mater., and Appl. Catal. B-Environ., holds over 20 authorized invention patents, and has led more than 10 national and provincial-level research projects, receiving awards such as the First Prize of the Golden Bridge Award from the China Technology Market Association and the First Prize of the Shandong Provincial Science and Technology Progress Award as the first contributor and main participant.
Professor Huifang Li: Professor at the School of Mechanical and Electrical Engineering, Qingdao University of Science and Technology, PhD supervisor. She obtained her PhD in theoretical and computational chemistry from Shandong University in June 2009 and then conducted postdoctoral research on computational simulations of optoelectronic functional materials in the group of Professor Jean-Luc Brédas, a European Academy member at Georgia Institute of Technology. In September 2019, she joined the School of Mechanical and Electrical Engineering at Qingdao University of Science and Technology. Her main research directions include quantum computational chemistry of functional materials and classical molecular dynamics simulations. Currently, she has published over 80 SCI research papers, with more than 50 as the first/corresponding author, including in top academic journals such as Chem. Mater., J. Mater. Chem. A, Adv. Mater., and ACS Nano. She has led two National Natural Science Foundation projects, an Education Ministry Overseas Study Fund project, a Shandong Provincial Key R&D project, a Shandong Provincial Natural Science Foundation project, a Jiangxi Provincial Natural Science Foundation project, and an open project from Xiamen University, with a total funding of over 2 million yuan. She has also participated in a key project of the National Science and Technology Support Program during the 11th Five-Year Plan (ranked third) and several international projects with Solvay in Europe, the U.S. Army Multidisciplinary Research Laboratory, and Saudi Aramco.
Transforming Waste into Treasure in 60 Seconds, Recovery Rate > 99.5%! A New Solution to Battery Recycling Challenges in Nat. Sustain.
2025-04-07

Significant Research in ACS Energy Lett: Correlation Study of “Oxygen Loss” and Capacity Decay of Nickel-Rich Cathodes
2025-04-07
Professor Wu Feixiang’s Team at Central South University: Heterogeneous Doping Constructs Multifunctional Colloidal Electrolytes for Ultra-Stable Operation of Lithium Metal Batteries
2025-04-07

Professor Wang Mingsheng at Xiamen University: Utilizing 3D Hosts to Regulate the Temporal and Spatial Order of Lithium Deposition and Stripping in Solid-State Batteries
2025-04-07

Professor Sun Yongming’s Team at Huazhong University of Science and Technology: Electron/Ion Mixed Conductive Agents Facilitate Fast Charging Performance of Thick Battery Electrodes
2025-04-07

Wang Jian/Lin Hongzhen/Zhang Yongzheng Adv. Mater.: Low-Temperature Zinc Metal Battery Suspended Electrolytes with Catalytic Self-Accelerating Desolvation Kinetics
2025-04-07

IF > 40 Top Journal Articles: Nickel-Based Layered Oxide Cathode Materials
2025-04-06

Professor Xie Jia’s Team at Huazhong University of Science and Technology: Synergistic Pre-Lithiation Strategy for High-Performance Lithium-Ion Batteries Throughout Their Lifecycle
2025-04-06

Ultrasonic Spectroscopy for Early Detection and Dynamic Monitoring of Lithium Deposition in Lithium-Ion Batteries
2025-04-06

Beijing Deep Research Institute’s Pan Feng/Jiangsu University’s Zhao Yan/University of Surrey’s Yang Kai AEM: In-Situ Amine Decomposition of FEC Assists Carbonate-Based Soft Pack Batteries to Stabilize 10C Fast Charging
2025-04-06