 Princeton University The team led by Daniel J. Cohen has developed a 3D structure smaller than a single cell, called the Self-Adhesive Tunnel (SAT), which allows cells to wrap around it and adhere to themselves. The related results were published in the journal “Engineering Cellular Self-Adhesions Inside 3D Printed Micro-Arches to Enhance Cell: Biomaterial Attachment“.
Princeton University The team led by Daniel J. Cohen has developed a 3D structure smaller than a single cell, called the Self-Adhesive Tunnel (SAT), which allows cells to wrap around it and adhere to themselves. The related results were published in the journal “Engineering Cellular Self-Adhesions Inside 3D Printed Micro-Arches to Enhance Cell: Biomaterial Attachment“.

Highlights: Innovation and Breakthrough
1. Design of Self-Adhesive Tunnel (SAT): This is the first proposal to utilize 3D printing technology to construct micro-nano scale self-adhesive tunnels, inducing cells to form adhesive structures to attach to biomaterials, breaking through the traditional reliance on extracellular matrix (ECM) or integrins for cell attachment.
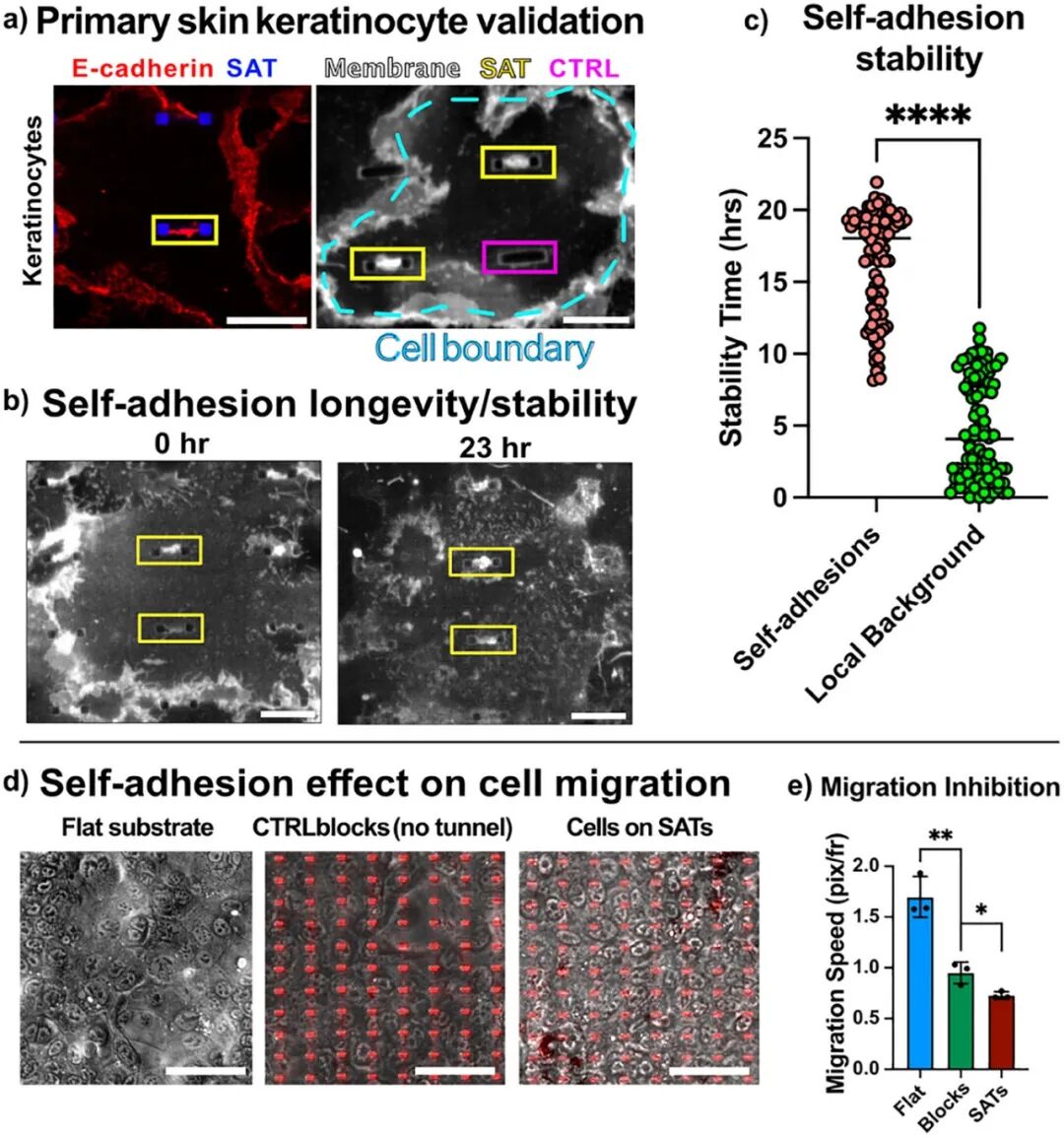
2. Validation Across Multiple Cell Types: The effectiveness of SAT has been validated in various cell types (renal cells, skin cells, stem cells, and endothelial cells), demonstrating the universality of this strategy.
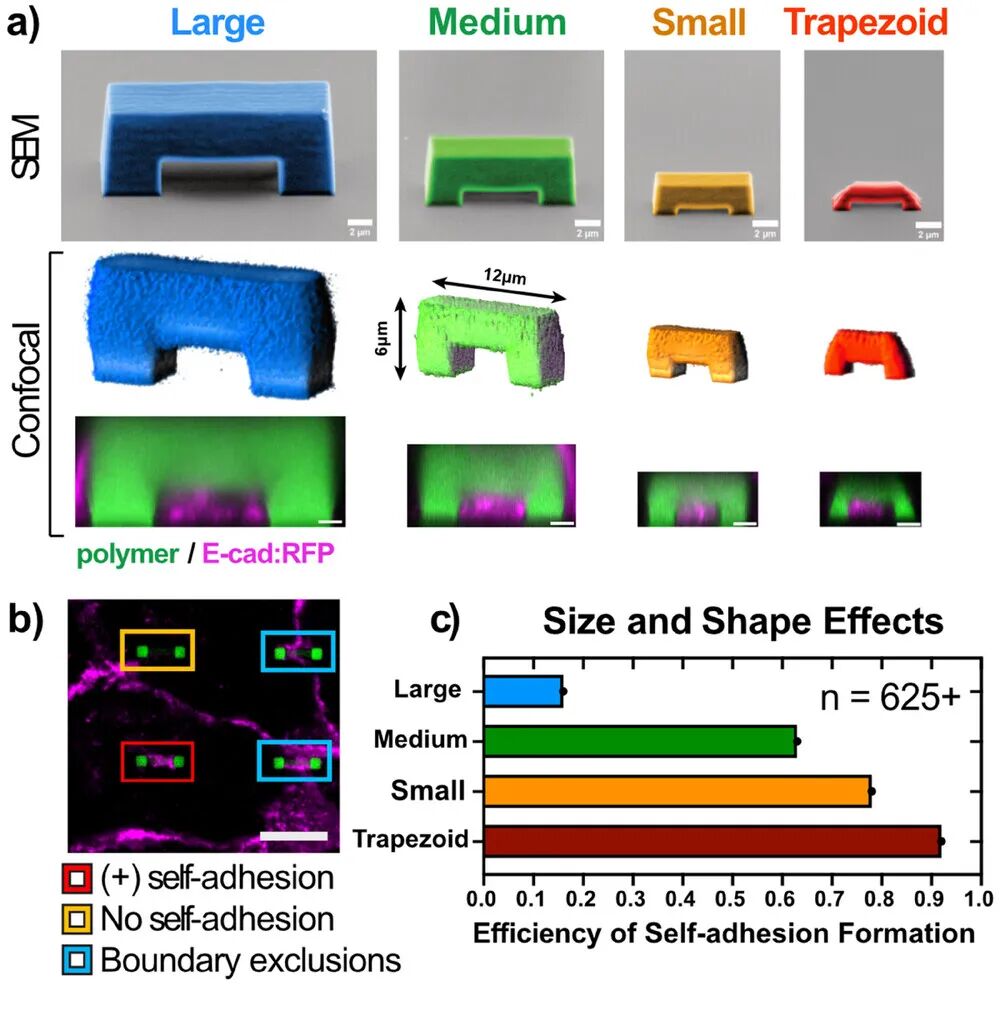
3. Efficient Self-Adhesion Formation: By optimizing the size and shape of SAT, the efficiency of self-adhesion formation was increased from approximately 65% to 93%, significantly enhancing the efficiency of cell-biomaterial binding.
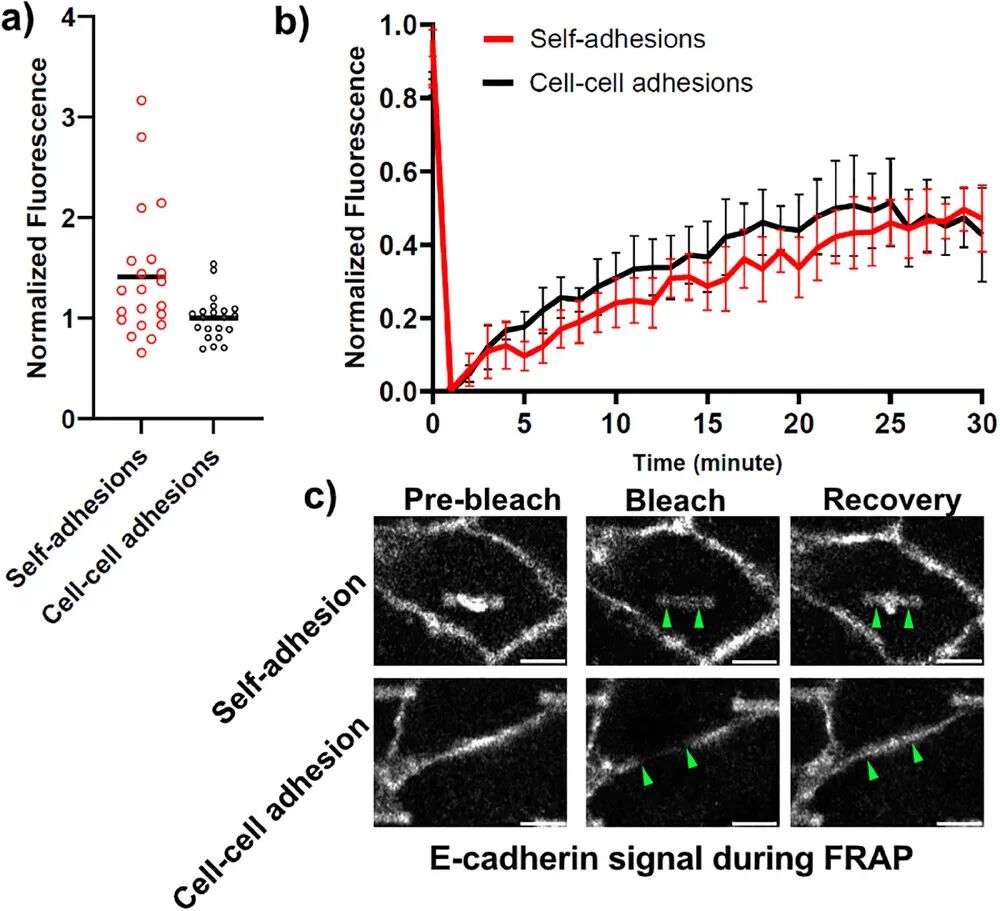
4. Long-term Stability: It has been demonstrated that the self-adhesive structure can stably exist in cells for over 96 hours, maintaining functionality during long-term culture, ensuring the long-term stability of biomaterials.
WHAT: Research Content
This research develops a novel strategy for cell-biomaterial binding, which involves 3D printing technology to manufacture micro-nano scale “self-adhesive tunnels” (SAT), inducing cells to form adhesive structures to attach to biomaterials. The research team significantly improved the efficiency of cell self-adhesion by optimizing the size and shape of SAT, and validated its effectiveness across various cell types (including renal cells, skin cells, stem cells, and endothelial cells). Additionally, the study explored the stability of self-adhesion and its impact on cell migration behavior, providing important theoretical and technical support for the design and application of future biomaterials.
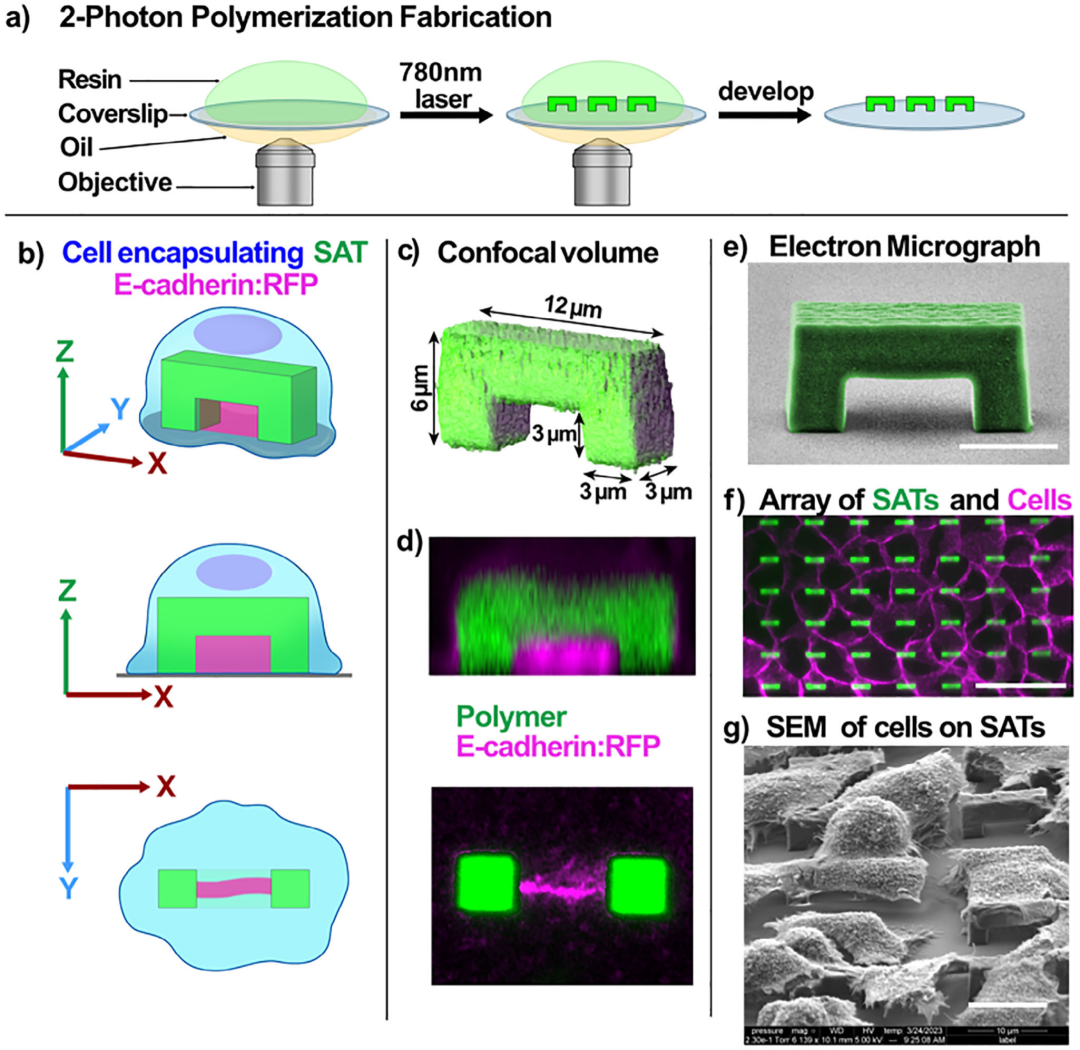
Figure 1 Manufacturing and Characterization of 3D Printed Self-Adhesive Tunnel (SAT) Arrays
WHY: Research Background and Significance
Traditional biomaterial design primarily relies on the interaction between cells and the extracellular matrix ( ECM), achieving cell attachment through receptors such as integrins. However, this strategy faces numerous issues at the interface between soft tissues and hard implants, such as infection, inflammation, and tissue encapsulation. Cell adhesion is mainly mediated by the cadherin family, which plays a crucial role in mechanical transduction, collective behavior, and tissue integrity. Therefore, developing a novel biomaterial binding strategy based on intercellular adhesion mechanisms can effectively address the limitations of existing technologies and provide new ideas for the integration of soft tissue implants and the design of tissue engineering scaffolds, promoting the development of the biomaterials field.
HOW: Research Methods
The research team achieved this innovation through the following methods:
1. 3D Printing Technology: Utilizing two-photon polymerization (2PP) based 3D nano-printing technology, micro-nano scale self-adhesive tunnels (SAT) were manufactured, with sizes smaller than a single cell, capable of inducing cells to form adhesive structures.
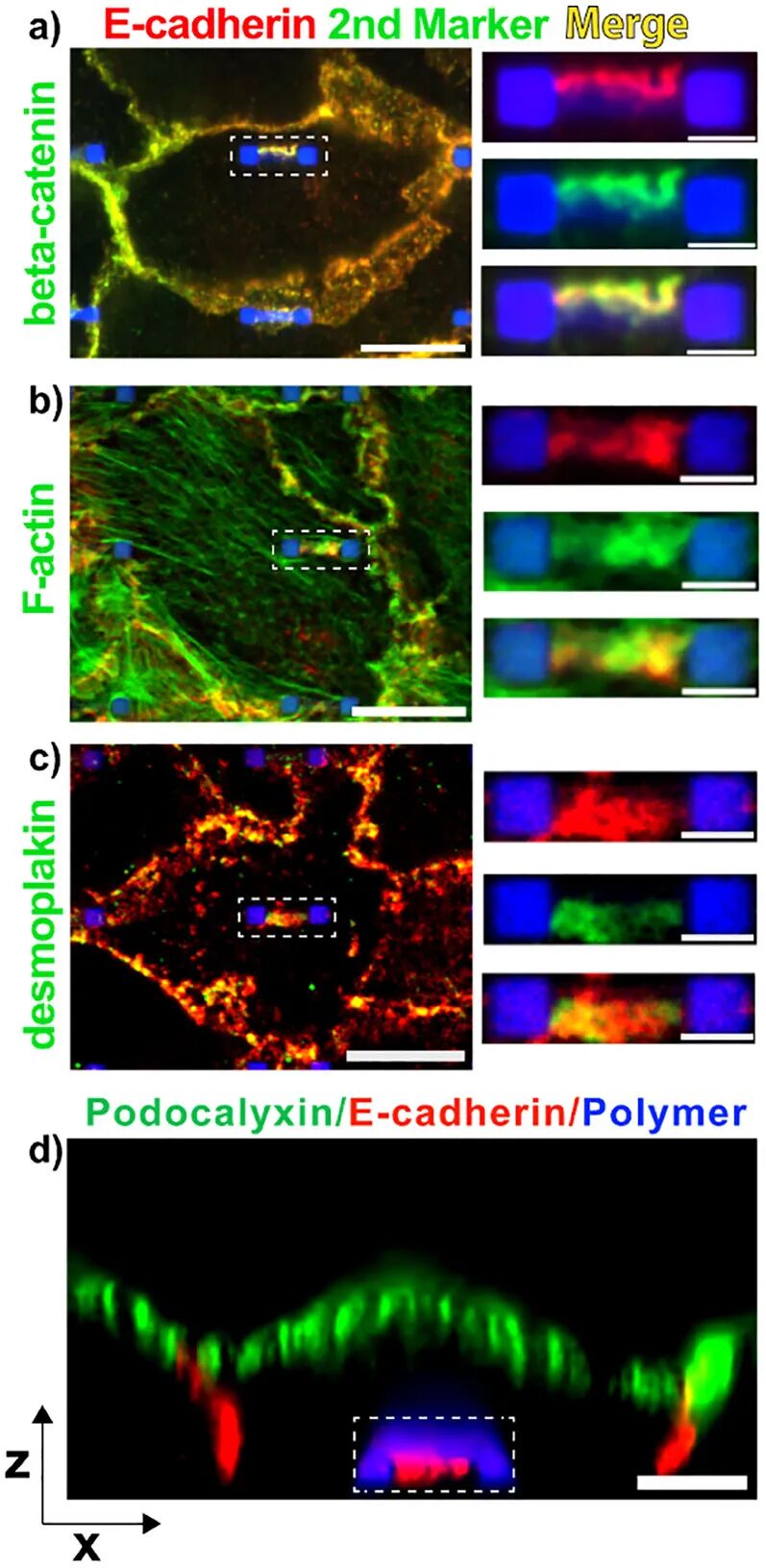
2. Cell Culture and Experiments: Various cell types (such as renal cells, skin cells, stem cells, and endothelial cells) were selected for experiments, observing the formation of self-adhesion by culturing cells on SAT, and verifying the stability and biocompatibility of self-adhesion through techniques such as fluorescence labeling.
3. Design Optimization: By altering the size and shape of SAT, parameter optimization experiments were conducted to determine the optimal SAT structure to enhance the efficiency of cell self-adhesion formation.
4. Biomechanical Analysis: Using fluorescence recovery after photobleaching (FRAP) technology, the kinetic differences between self-adhesion and normal intercellular adhesion were studied to assess stability.
5. Cell Migration Experiments: Long-term cell migration experiments were conducted to analyze the impact of SAT on cell migration behavior, verifying its potential application value in biomaterial binding.
Solutions: Technical Advantages
1. High Efficiency: By optimizing the size and shape of SAT, the efficiency of cell self-adhesion formation was significantly improved, reaching up to 93%, far exceeding traditional methods.
2. Biocompatibility: The self-adhesion induced by SAT can stably exist for over 24 hours, and cells exhibit normal growth and migration behavior on SAT, demonstrating good biocompatibility.
3. Universality: The research demonstrated that the SAT strategy effectively induces self-adhesion across various cell types (including renal cells, skin cells, stem cells, and endothelial cells), showing broad applicability.
4. Stability: The self-adhesive structure significantly reduces cell migration speed, helping to stabilize the interface between cells and biomaterials, ensuring the long-term stability and functionality of soft tissue implants.
Conclusion: Future Prospects
The cell-biomaterial binding strategy based on self-adhesive tunnels (SAT) proposed in this article provides a new approach to addressing the stability issues at the interface between soft tissues and hard implants. By further optimizing the design and manufacturing processes of SAT, it is expected to develop more efficient and stable biomaterial interfaces. Future research can explore the application of SAT in three-dimensional tissue models and its actual effects in clinical implants, opening new directions for the development of the biomaterials field.
✦
•
✦
✦
Swipe left and right to see more
✦
Source: https://doi.org/10.1002/adma.202502425
Author: Lin Yunhai
Reviewer: Liu Yaolin
Final Reviewer: Chao Yanxia
Follow us for more updates