 Layered double hydroxides (LDHs) based on transition metals have demonstrated high efficiency as catalysts in the oxygen evolution reaction (OER) during alkaline water electrolysis, which is crucial for sustaining hydrogen production. However, there is an urgent need to explore new LDH-based electrocatalysts that possess both high activity and good stability, which is essential for a comprehensive understanding and significant improvement of the sluggish OER kinetics. Here, we designed and prepared a series of bimetallic (cobalt and molybdenum) LDH arrays through a simple and controllable strategy. This strategy involves incorporating a molybdenum source into a pre-synthesized cobalt-based metal-organic framework (MOF) array (named ZIF-67/CC array) on carbon cloth (CC). We found that by adjusting the molybdenum content, the resulting catalyst exhibited gradual differences in structural characteristics, surface morphology, and chemical state, resulting in CoMox-LDH/CC catalysts (where x represents the weight of the added molybdenum source). Encouragingly, the best-performing CoMo0.20-LDH/CC electrocatalyst exhibited a low overpotential of only 226 mV and high stability at a current density of 10 mA·cm⁻², outperforming most previously reported LDH-based OER catalysts. Additionally, a voltage of only 1.611 V is required to drive the overall water splitting device at a current density of 10 mA·cm⁻². This study represents a significant advancement in the development and application of new OER catalysts.
Layered double hydroxides (LDHs) based on transition metals have demonstrated high efficiency as catalysts in the oxygen evolution reaction (OER) during alkaline water electrolysis, which is crucial for sustaining hydrogen production. However, there is an urgent need to explore new LDH-based electrocatalysts that possess both high activity and good stability, which is essential for a comprehensive understanding and significant improvement of the sluggish OER kinetics. Here, we designed and prepared a series of bimetallic (cobalt and molybdenum) LDH arrays through a simple and controllable strategy. This strategy involves incorporating a molybdenum source into a pre-synthesized cobalt-based metal-organic framework (MOF) array (named ZIF-67/CC array) on carbon cloth (CC). We found that by adjusting the molybdenum content, the resulting catalyst exhibited gradual differences in structural characteristics, surface morphology, and chemical state, resulting in CoMox-LDH/CC catalysts (where x represents the weight of the added molybdenum source). Encouragingly, the best-performing CoMo0.20-LDH/CC electrocatalyst exhibited a low overpotential of only 226 mV and high stability at a current density of 10 mA·cm⁻², outperforming most previously reported LDH-based OER catalysts. Additionally, a voltage of only 1.611 V is required to drive the overall water splitting device at a current density of 10 mA·cm⁻². This study represents a significant advancement in the development and application of new OER catalysts.
01 Research Background and Objectives
Background: Layered double hydroxides (LDHs) based on transition metals serve as efficient catalysts in the alkaline oxygen evolution reaction (OER), which is vital for sustaining hydrogen production.
Objective: To explore new LDH-based electrocatalysts to enhance the activity and stability of OER, thereby improving OER kinetics.

02 Research Methods
-
A series of cobalt-molybdenum bimetallic LDH arrays were designed and fabricated by introducing a molybdenum source into a pre-synthesized cobalt-based metal-organic framework (MOF) array (named ZIF-67/CC array).
-
By adjusting the molybdenum content, catalysts with different molybdenum contents were prepared, named CoMox-LDH/CC (where x represents the weight of the added molybdenum source).
03 Visual Guide
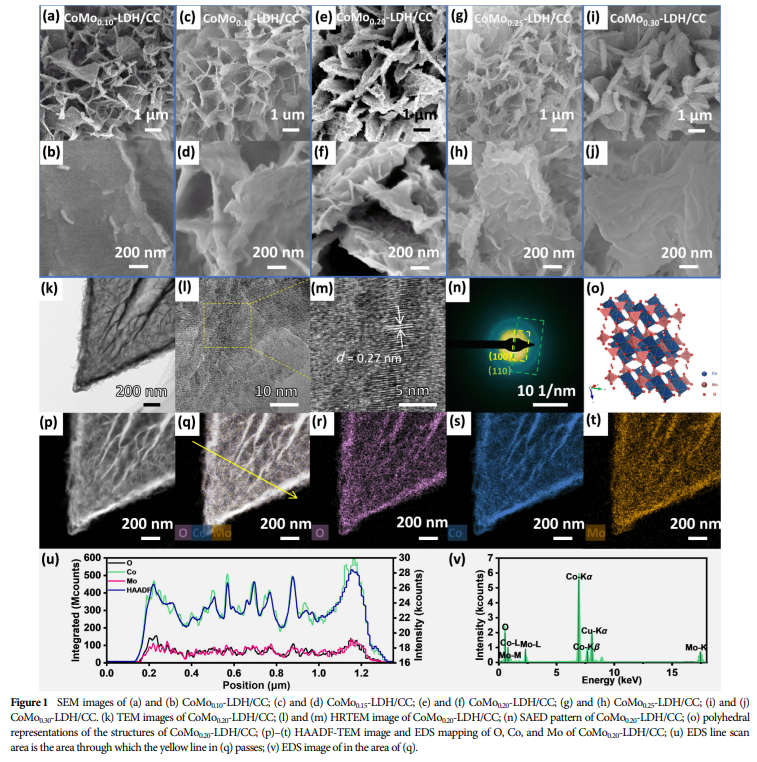
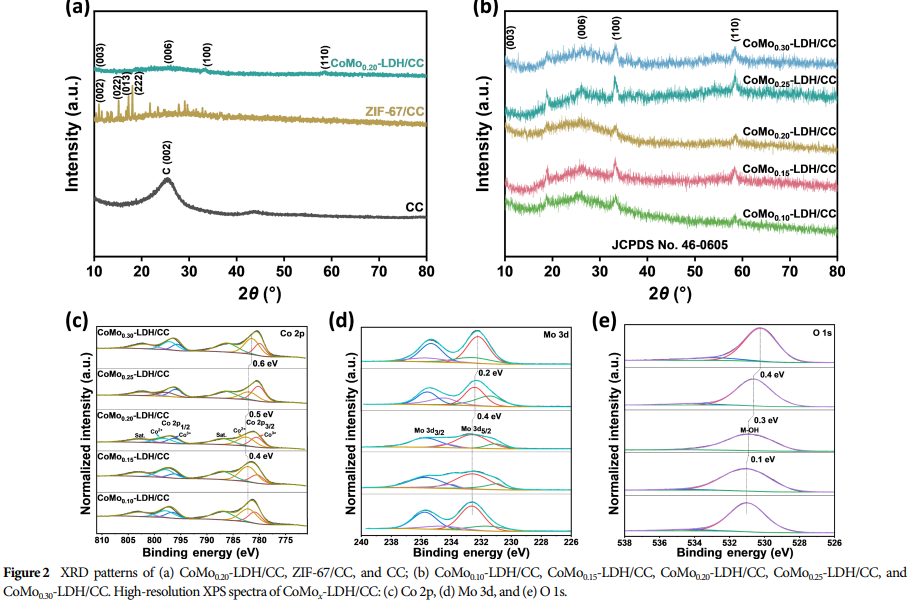
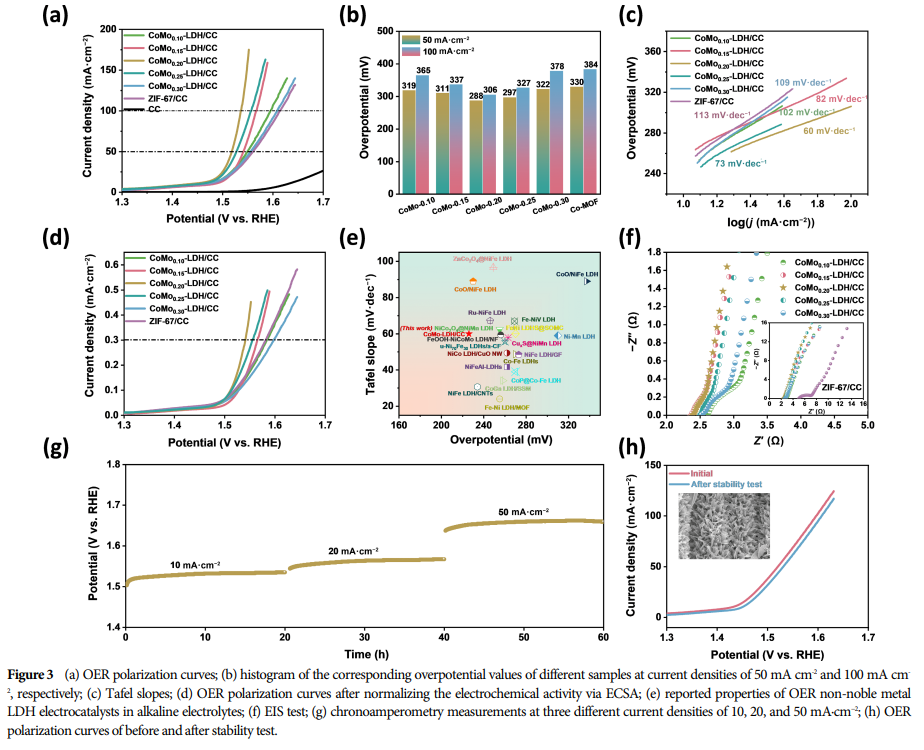

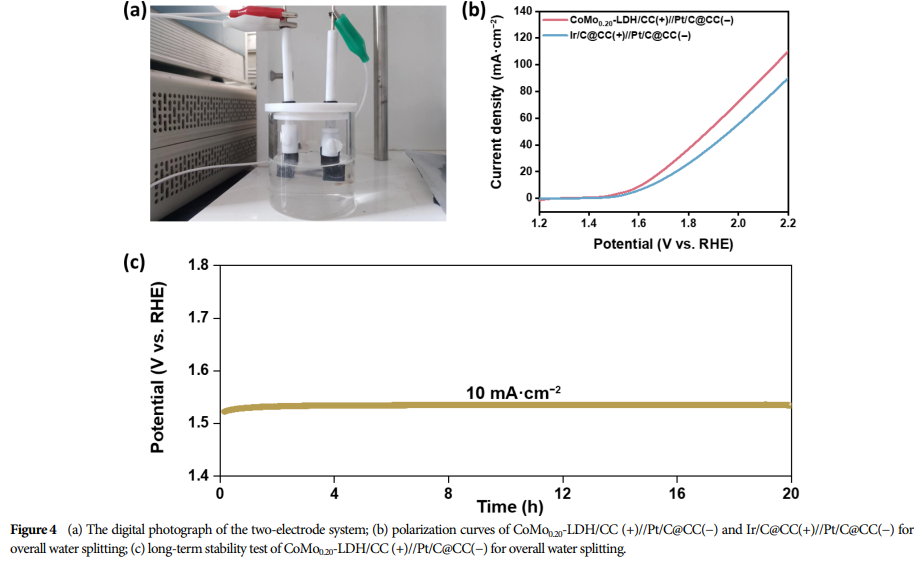
04 Research Results
-
Adjusting the molybdenum content led to gradual differences in the structural properties, surface morphology, and chemical state of the catalysts.
-
The best-performing CoMo0.20-LDH/CC electrocatalyst exhibited a low overpotential of only 226 mV and high stability at a current density of 10 mA·cm−2.
-
This catalyst requires only a voltage of 1.611 V to achieve a current density of 10 mA·cm−2 when driving the overall water splitting device.
05 Research Significance
-
The performance of the CoMo0.20-LDH/CC catalyst surpasses that of most previously reported LDH-based OER catalysts.
-
This study represents an important advancement in the development and application of OER catalysts.
06 Future Research Directions
-
Further optimization of the catalyst composition and structure to enhance its activity and stability.
-
Exploration of the catalyst’s application potential in larger-scale water electrolysis devices.
-
Research on the regeneration and recycling mechanisms of the catalyst to reduce production costs and environmental impact.
07 Conclusion
-
This study successfully enhanced the efficiency of the oxygen evolution reaction in alkaline water electrolysis by designing and fabricating cobalt-molybdenum bimetallic LDH arrays.
-
The CoMo0.20-LDH/CC catalyst exhibited excellent performance and stability, providing new directions for the development and application of OER catalysts.
-
However, future research must still focus on the long-term stability and cost-effectiveness of the catalyst to promote its widespread adoption in practical applications.
This article is provided by the Hydrogen Energy Research Assistant.Original link:https://www.sciopen.com/article/10.1007/s12274-024-6529-1▲ Programmable High-Precision Desktop Membrane Electrode Spraying Machine