
Medical Device Inspection Services:
-
Registration Inspection, Preliminary Testing, Rectification Services
-
Biocompatibility, Product Shelf Life and Packaging, Sterilization Testing, Animal Testing
-
Electrical Safety Testing, Electromagnetic Compatibility Testing and Rectification
-
Physical Properties of Materials, Chemical Analysis, Biological Performance Testing
-
Performance Testing of Semi-finished and Finished Products, Reliability Testing
-
Formula Analysis, Formula Restoration
-
Failure Analysis……
National Customer Service Phone: 400-818-0021
Add customer service WeChat anytesting001 to receive daily medical device news
For more technical content, please visit: Medical Device R&D Column
http://med.anytesting.com
The following is the main text:
Research Scope
The authors conducted a comprehensive study to evaluate the performance of flexible blister packaging made from DuPont™ Tyvek® 2FS™, Tyvek® 40L, and two commonly used medical papers (greater than 80 grams medical dialysis paper and 60 grams direct-seal medical dialysis paper) formed – filled – heat sealed (FFS). Approximately 2,800 packages were tested in two phases of the study.
In the first phase of the study, the thermal sealing process window range for all four blister packaging material combinations was defined, and the optimal process parameters for producing the four types of packaging were determined. All four material combinations met the specified requirements (minimum heat seal strength, seal integrity, peel performance, and visual inspection requirements), but there were some differences in heat seal strength performance. The white paper covering the first phase of the study was published in October 2018.
After completing the basic assessment, flexible blisters were processed according to the defined nominal process parameters and used to package selected medical devices (blood transfusion devices), followed by sterilization (double-cycle ethylene oxide sterilization or gamma radiation sterilization). Packaging testing was conducted by an independent and certified laboratory according to the standards listed in ISO 11607.
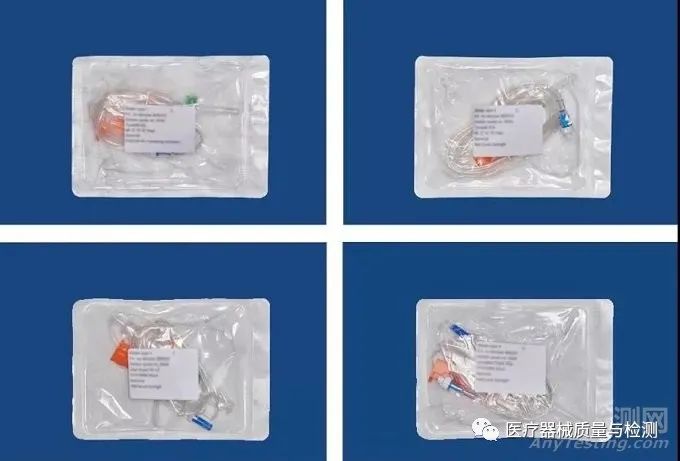
Figure 1. Sample of packaging for blood transfusion devices
Integrity and strength tests of the packaging were conducted before and after sterilization; environmental standard condition simulations and subsequent pallet simulation transport tests (Sequence A); as well as humid environmental condition simulations and subsequent carton simulation transport tests (Sequence B). The purpose of Sequence B was to assess whether high humidity conditions could negatively affect packaging materials and heat seals, potentially increasing the risk of sterility breaches. Standard statistical methods were applied to define the sample size.
Packaging Integrity
The packaging integrity report indicated that three of the four tested blister material combinations failed the tests. Several blisters made from medical paper (greater than 80 grams medical dialysis paper and 60 grams direct-seal medical dialysis paper) exhibited bubble leakage tests (ASTM F2096) after standard environmental simulation (Sequence A) and transport tests under humid environmental conditions (Sequence B). One blister made from Tyvek® 40L reported a failure after Sequence B testing, while all blisters made from Tyvek® 2FS™ passed.
The integrity test results indicated that increasing the weight of the paper does not necessarily reduce the risk of packaging failure. Moisture or humid environmental conditions negatively affect the integrity of the packaging after transport, especially for cellulose-based materials. (The integrity test failures for the above blisters occurred only after gamma radiation sterilization and transport testing.)

Figure 2. Example of integrity failure in medical paper blister packaging
All samples of the four blister material combinations passed the dye penetration test, confirming the integrity of all blister heat seals.
Visual Inspection
Visual inspection of the color stability and heat seal quality of the packaging materials. Among the four tested blister material combinations, two types of medical paper showed varying degrees of yellowing after gamma radiation sterilization. This indicates that gamma radiation negatively affects the appearance of the paper, likely related to the degradation of cellulose or other components. Blisters made from Tyvek® 40L or Tyvek® 2FS™ showed no noticeable discoloration.

Figure 3. Color changes in medical paper blister packaging after sterilization
According to ASTM F1886 testing method, all blister packaging heat seals were inspected before and after sterilization, with no abnormalities reported.
Packaging Strength Analysis
The heat seal strength results (ASTM F88) of each blister material combination were verified to determine whether sterilization (ethylene oxide or gamma irradiation) and humid environmental simulations, as well as subsequent transport tests, affected the data trends.
Heat seal strengths for Tyvek® 40L and Tyvek® 2FS™ blisters ranged between 3 N / 15 mm and 4 N / 15 mm, showing normal variability, and the heat seal strength remained stable after sterilization under each condition.
Heat seal strength for blisters made from greater than 80 grams medical dialysis paper was at the same level but exhibited a wider range of variability, with further increases in variability observed after sterilization and under humid conditions during subsequent transport tests.
Compared to other blister material combinations, the heat seal strength of the 60 grams direct-seal medical dialysis paper blisters was the lowest (approximately 2 N / 15 mm). This affected the data trends.
Verification analysis of burst strength results (ASTM F2054) for each blister material combination was performed to determine whether sterilization (gamma radiation sterilization) and/or humid environmental conditions and subsequent transport tests could potentially affect data trends.
Compared to other blister material combinations, Tyvek® 2FS™ blister packaging exhibited the highest burst strength, while the 60 grams direct-seal medical dialysis paper blister had the lowest burst strength. All blister material combinations, except for Tyvek® 40L blisters, showed a reduction in burst strength after sterilization and humid environmental simulations, as well as subsequent transport tests.
Burst Testing
During transport, pressure changes due to altitude and temperature variations may increase the risk of sterility breaches in the packaging. Experiments based on the modified burst strength testing of ASTM F1140 demonstrated that high permeability positively impacts the burst strength of the packaging.
This experiment clearly demonstrated the benefits of high permeability packaging, such as those made from Tyvek® 40L, which reduced the risk of package rupture during transport and handling.
Conclusion
Based on the results of Phase 2 of the study, it can be concluded that a risk-based packaging performance testing plan (e.g., including humid environmental simulations before transport tests) is essential to ensure that all device packaging meets requirements, such as compliance with the new MDR and ISO 11607 standards. Test conditions should be selected based on risk assessments that consider all potential challenges throughout the lifecycle of the device and packaging, from manufacturing to use. It is crucial to consider all aspects of risk, such as transport or extreme environmental conditions and/or sterilization methods and doses, to determine appropriate packaging materials and designs. It is strongly recommended to conduct pre-screening tests before starting any validation work to avoid costly re-validation and commercialization delays.
Authors: Nicole Kaller, Laetitia Clerc, Zhu Xueyan, Qin Lei,
DuPont (China) R&D Management Co., Ltd.
Medical Device Packaging Testing, Simulation Transport Testing
National Customer Service Phone: 4008180021
Massive medical device R&D materials available for download:
Part of the Directory
-
(Chinese version) Medical Device R&D Handbook – Second Edition – Part Two: Processes in Medical Device R&D
-
(Chinese version) Medical Device R&D Handbook – Second Edition – Part Three: Methods in Medical Device R&D
-
(Chinese version) Medical Device R&D Handbook – Second Edition – Part Four: Interviews and Highlights with R&D Entrepreneurs
-
(Chinese version) Medical Device R&D Handbook – Second Edition – Part One: Materials in Medical Device R&D
-
DFMA Design for Manufacturing and Assembly English Version Parts 11-12 (98 pages)
-
DFMA Design for Manufacturing and Assembly English Version Parts 1-2 (111 pages)
-
DFMA Design for Manufacturing and Assembly English Version Parts 13-14 (113 pages)
-
DFMA Design for Manufacturing and Assembly English Version Parts 3-4 (112 pages)
-
DFMA Design for Manufacturing and Assembly English Version Parts 5-6 (76 pages)
-
DFMA Design for Manufacturing and Assembly English Version Parts 7-8 (111 pages)
-
DFMA Design for Manufacturing and Assembly English Version Parts 9-10 (86 pages)
-
[E-Book] Guidelines for Thermal Design of Electronic Equipment by Xu Weixin (193 pages)
-
[E-Book] Product Design for Manufacturing and Assembly (567 pages) Chinese version
-
[E-Book] Design, Execution and Management of Clinical Trials for Medical Devices (296 pages)
-
2020 China Allergen Product R&D and Industry Research Report (16 pages)
-
570 Common Standards for Medical Device R&D Summary.doc (40 pages)
-
DFMA Design for Manufacturing and Assembly.ppt (30 pages)
-
DFMEA Development and Production.ppt (53 pages)
-
DOE Experimental Design and Practical Operation Training PPT
-
DOE Experimental Design Template.xls
-
ESD Protection Design and Handling Training PPT (26 pages)
-
FDA Released Tablet Development QBD Case (92 pages)
-
FDA Medical Device Manufacturer Design Control Guidance (28 pages)
-
Application Training PPT of GB 9706.1-2020 in Medical Device R&D Process (61 pages)
-
GMP Independent Software Quality System Design and Development Explanation (Part 1) PPT (22 pages)
-
IEC60601-1 Medical Device Safety Standard Principles and Design Training Materials.ppt (116 pages)
-
IEC61215.2005 Ground-mounted Crystalline Silicon Photovoltaic Modules – Design Verification and Qualification GB/T 9535 (Chinese version, 34 pages)
-
IND, NDA Dissolution Method Development Confirmation Training PPT (50 pages)
-
LC-MS Liquid Chromatography Mass Spectrometry Method Development
-
PCBA Circuit Board Level Reliability Design (Single Board, Components).ppt (25 pages)
-
PCB Design Basic Process Requirements (5 pages)
-
TI Power Supply Design Experience Collection (98 pages)
-
TI Design: Reference Design for Weight Scale with Human Composition Function and BLE Connectivity
-
USP 1092 Dissolution Test Development and Validation [Bilingual Edition].doc (54 pages)
-
Ankai Bus R&D Quality Management System Training Materials.ppt (60 pages)
-
Design and Development Failure Mode Analysis Case of Safety Blood Collection Needle.doc (3 pages)
-
Sheet Metal Structure Design Process Handbook (36 pages)
-
Portable Heart Rate Collection System Design.doc(40 pages)
-
Design and Development Procedures for Products and Services (including forms).doc (21 pages)
-
Product Structure Design Guidelines – Draft Angle.doc (3 pages)
-
Product Structure Design Guidelines – Holes.doc (4 pages)
-
Product Structure Design Guidelines – Tolerances.doc (4 pages)
-
Product Structure Design Guidelines – Snap Joints.doc (6 pages)
-
Product Development Process PPT (12 pages)
-
Product R&D Management System (17 pages)
-
Testing Training PPT in Product R&D Process
-
Defibrillator R&D and Industry Research Report (34 pages)
-
Innovative Drug R&D Process Training.ppt
-
Vendor Management Research in the R&D Process of Large Medical Equipment – A Case Study of GE Medical.doc (52 pages)
-
Electromagnetic Compatibility Design Training Materials.ppt (83 pages)
-
Electronic System Reliability Design Training PPT (129 pages)
-
Trends in Electronic Blood Pressure Monitor Product Design.ppt
-
Design Guidelines for Error-proofing Training Materials.ppt (34 pages)
-
Generic Drug Development Template Example
-
Overview of Generic Drug R&D Project Management System Training PPT (37 pages)
-
Tooling and Fixture Design Training Lecture PPT (80 pages)
-
Selection Criteria for Design Verification and Confirmation Based on Risk (6 pages)
-
Information on the R&D, Registration, and Testing of In Vitro Diagnostic Reagents Part 1 (24 pages)
-
Information on the R&D, Registration, and Testing of In Vitro Diagnostic Reagents Part 2 (9 pages)
-
Huawei EMC Design Guidelines (85 pages)
-
Huawei PCB Design Specifications (21 pages)
-
Huawei Electromagnetic Compatibility Structural Design Specifications (56 pages)
-
Huawei Electromagnetic Compatibility Structural Design Specifications V20 (133 pages)
-
Huawei Protection Circuit Design Specifications (60 pages)
-
Huawei Process Reliability Design Methods and Practices.ppt (71 pages)
-
Huawei Thermal Design Training PPT (92 pages)
-
Huawei R&D Department – Product Structure Design and Mold Development Process (6 pages)
-
Huawei R&D Requirements and Design Engineering Document Writing Training (including templates).ppt (90 pages)
-
Chemical Raw Material Drug Process R&D and Process Validation.ppt
-
Mechanical Reliability Design Training PPT (240 pages)
-
Mechanical Design Terminology Chinese-English Comparison (24 pages)
-
Research on R&D Team Performance Evaluation Model Based on Product Development Process (3 pages)
-
Research and Design of Body-Emitting Coils Based on Magnetic Resonance Imaging.caj (67 pages)
-
Development Strategy for Impurities in Drug Research and Analysis Methods Based on Risk Assessment & Common Problems in Impurity Research.ppt (76 pages)
-
Design and Realization of Ultrasonic Nebulizer Based on Embedded System.caj (61 pages)
-
Emergency R&D Management Process
-
Switching Power Supply Design A to Z (523 pages)
-
Lightning Surge Protection Design Technology Training Materials.ppt (64 pages)
-
Medtronic Puritan Bennett 560 (PB 560) Ventilator Complete Design Data (1)
-
Medtronic Puritan Bennett 560 (PB 560) Ventilator Complete Design Data (2)
-
Design Guidelines for Assembly Training Materials.ppt (81 pages)
-
Mold Design and Processing Technology.doc (31 pages)
-
Chery Automobile Whole Vehicle Development Process.ppt
-
Automobile Parts Development Process (3 pages)
-
Discussion on the Application of HALT Testing in Reliability Design
-
Discussion on the Necessity of Reliability Testing in Product Design Verification of Measurement and Control Devices
-
Discussion on the Design and Development Transition of Medical Devices
-
Key Points in Balloon Pump Design – Interpretation of YY/T0450.3-2016 Standard.doc (5 pages)
-
Trends and Opportunities Facing New Drug R&D under Globalization (6 pages)
-
Complete Medical Device Development Documents
-
Complete Medical Device Design and Development Materials (modifiable templates).doc (95 pages)
-
Thermoplastic Elastomer Formula Design Tips (6 pages)
-
Current Status and Development of Artificial Heart Valves (4 pages)
-
How to Manage Drug R&D Records and Data.doc (35 pages)
-
How to Conduct R&D for Raw Materials.ppt (77 pages)
-
How to Design Effective Preclinical Animal Efficacy Experiments for Implantable Medical Devices.ppt (25 pages)
-
Software Design and Development Control Procedures.doc(10 pages)
-
Design Scheme for Three-Way Valve and PC Embedded Adhesive.doc (14 pages)
-
Sharing on Design and Development Verification
-
Design and Development Verification Phase
-
Design Development Form Template (36 pages)
-
Design Failure Mode and Effects Analysis (DFMEA)
-
Classic Case Analysis of Design Failure Analysis DFMEA.ppt (41 pages)
-
Full Process of R&D for Biological and Medical Materials.ppt (10 pages)
-
Application of Experimental Design in the Confirmation of Medical Device Production Process (8 pages)
-
Design of Externally Charged Implantable Artificial Cardiac Pacemaker (4 pages)
-
Key Points and Statistical Analysis Methods in the Design of In Vitro Diagnostic Reagents Training PPT (88 pages)
-
Design and Development of In Vitro Diagnostic Reagents to Market Training PPT (191 pages)
-
Design Requirements for Coating Dust High-Temperature Ovens on Ducts and Heating
-
Design and Preparation of External Ointments Training Materials PPT (35 pages)
-
Sterile Medical Device Packaging Design Development and Experiment Training PPT (74 pages)
-
Sterile Medical Device Packaging Design Training PPT (35 pages)
-
Guidelines for Writing Technical and Design Documents for Passive Medical Devices (25 pages)
-
Principles and Training Materials for Modern Medical Electronic Instrument Design.ppt (58 pages)
-
Analysis and Design Ideas for Defibrillation Testing of ECG Monitors (3 pages)
-
New Product Design and Development Process and Output Documents (4 pages)
-
Medical Device Design and Development under New Regulations.ppt
-
Design and Prototype Development of New Dental X-ray Machines (3 pages)
-
Key Points of Pharmacological and Toxicological Research in New Drug R&D Training Materials.ppt (46 pages)
-
New Drug R&D and Design Process.ppt (29 pages)
-
Design, Production, and Testing of Vascular Stents.ppt
-
Current Status and Outlook of Hemodialyzer Membrane Material R&D (4 pages)
-
R&D Department Operation Manual (164 pages)
-
R&D Management Process Specification.ppt
-
Assessment and Incentives for R&D Personnel.ppt (136 pages)
-
R&D Project Management – IPD Process Management.ppt
-
R&D Project Management Tools and Templates.ppt (134 pages)
-
R&D Project Completion Management Methods.doc (3 items)
-
Basic Training PPT on R&D Quality Management (24 pages)
-
Training PPT on R&D Quality Management (120 pages)
-
Types of Liquid Chromatography Columns and Method Development Training Materials.ppt (42 pages)
-
Clear Design and Development Flowchart.pdf
-
One Image to Understand the Requirements for Design and Development of Medical Device Systems
-
Brief Discussion on Medical Device Packaging Design (4 pages)
-
Summary of Design and Development Process, Documents, and Forms for Medical Device Products (56 pages)
-
Training PPT on Compliance Assurance in the Medical Device Product Development Process
-
Division of Stages in Medical Device Product Development and Main Tasks of Each Department.doc
-
Trends in Medical Device Product Design Development (8 pages)
-
Medical Device Product Design Development Plan.doc (2 pages)
-
Training Materials PPT on Sample Size Design for Medical Device Sampling Inspection
-
Three Major Barriers Faced by Medical Devices from R&D to Market PPT (45 pages)
-
Design Development and Technical Document List for Medical Devices
-
Analysis of Design Principles for Medical Devices (8 pages)
-
Common Problems and Design Rectification Training PPT for Electromagnetic Compatibility Testing of Medical Devices (131 pages)
-
Training PPT on Animal Experiment Research Design for Medical Devices (19 pages)
-
On-site Inspection Training PPT for Independent Software Design and Development of Medical Devices (22 pages)
-
Flowchart of Medical Device Development and Registration Process (2 pages)
-
Template for Medical Device Project Proposal and Design Development Plan Document (11 documents).doc
-
Principles and Design Methods for Medical Device Clinical Trial Protocols (3 pages)
-
Key Points in Designing Medical Device Clinical Trial Protocols Training PPT (24 pages)
-
Discussion on Key Points in Designing Medical Device Clinical Trial Protocols (6 pages)
-
Common Problems and Design Optimization Training PPT for Sterilization Packaging of Medical Devices (23 pages)
-
Design of Capability Verification Plans for Medical Devices (5 pages)
Medical Device Software User Interface Design Considerations.doc(3 pages)
Training PPT on Medical Device Design and Development Management (40 pages)
Medical Device Design and Development Process Control Training PPT
Technical Document List for Medical Device Design and Development (95 pages)
Medical Device Design and Development Control Procedures.doc (8 pages)
Medical Device Design Transition Procedures Document.doc (3 pages)
Medical Device Design Transition Report Template.doc (2 pages)
Medical Device Design Transition Training Materials.ppt (15 pages)
Discussion on Layout Design for Sterile Testing Rooms in Medical Device Manufacturing Companies.doc (3 pages)
Interpretation of Quality System Management Specifications for Medical Device Manufacturing – Design Development Training PPT(24 pages)
Flowchart of Medical Device Project Development Process.doc (3 pages)
Management Process for New Product Development in Medical Devices.doc (3 pages)
New Product Development Management System for Medical Devices.doc (7 pages)
Selection Criteria Table for New Product Development in Medical Devices.xls
New Product Design Development Process and Output Documents for Medical Devices.doc (4 pages)
Three Levels of Medical Device R&D.ppt
DMR and DHF Documents Required at Each Stage of Medical Device R&D.xlsx (4 pages)
Tools for Medical Device R&D
Comparison Table of National, Provincial, and Municipal Application Requirements for Medical Device R&D Institutions.xls
Full Cycle Document Directory for Medical Device R&D to Market.doc (3 pages)
Medical Device R&D Design (141 pages)
Medical Device R&D Handbook Second Edition – Part Two (Chinese version)
Medical Device R&D Handbook – Second Edition – Part One (Chinese version)
Medical Device R&D Document Management Specifications.doc (9 pages)
Design Control Guidelines for Medical Device Manufacturers (53 pages)
Design Qualification (DQ) Verification Plan Template for Medical Equipment.doc (15 pages)
Stainless Steel Materials & Special Alloy Materials in Medical Device R&D.ppt (15 pages)
Pharmaceutical R&D Analytical Method Validation Process Training PPT
Common Problems and Specifications for Original Records in Pharmaceutical R&D PPT
Considerations for Electromagnetic Compatibility Testing and Design of Medical Electrical Equipment (8 pages)
Progress in Medical Balloon R&D (9 pages)
Design, Preparation, and Testing of Medical Hydrogels.ppt (44 pages)
Current Status of Silver-based Antibacterial Materials and Their R&D Applications (3 pages)
Hardware Reliability Testing Design (5 pages)
Research on Malignant Ventricular Arrhythmia Discrimination Method for AED and Initial System Control Software Design.caj (64 pages)
Transparent SOUP and COTS Software for Medical Device Development (10 pages)
Raw Material Drug Process R&D and Control.ppt (90 pages)
Application of Orthogonal Experimental Design in Medical Device Design and Development Process (4 pages)
Stent Design
Regulatory Requirements Training Materials for Design and Development of Implantable Medical Devices.ppt (26 pages)
Basic Training Materials for Instrument Design.ppt (60 pages)
Draft for Product Development of Intelligent Nursing Machines.ppt(24 pages)
Wall Thickness Design Guidelines for Injection Molding Parts.doc (7 pages)
Final Sterilization Medical Device Packaging Solutions, Design Points, and Risk Assessment Training Materials.ppt (31 pages)