
Gas sensors are the core of gas detection systems and are typically installed within the probe head. Essentially, a gas sensor is a converter that transforms the volume fraction of a specific gas into a corresponding electrical signal. The probe head conditions the gas sample through the gas sensor, usually involving the filtration of impurities and interfering gases, drying or cooling treatment, sample suction, and even chemical treatment of the sample to enable faster measurements by the chemical sensor.

There are many types of gases with varying properties; thus, there are also many types of gas sensors. Based on the nature of the gas to be detected, sensors can be categorized into: sensors for detecting flammable and explosive gases, such as hydrogen, carbon monoxide, gas, gasoline vapors, etc.; sensors for detecting toxic gases, such as chlorine, hydrogen sulfide, arsenic, etc.; sensors for detecting gases in industrial processes, such as oxygen in steelmaking furnaces and carbon dioxide in heat treatment furnaces; sensors for monitoring atmospheric pollution, such as NOx, CH4, O3 that contribute to acid rain and household pollutants like formaldehyde. Based on the structure of the gas sensor, they can be divided into dry and wet types; based on the sensor’s output, they can be classified into resistive and non-resistive types; based on the detection methods, they can be categorized into electrochemical, electrical, optical, and chemical methods.
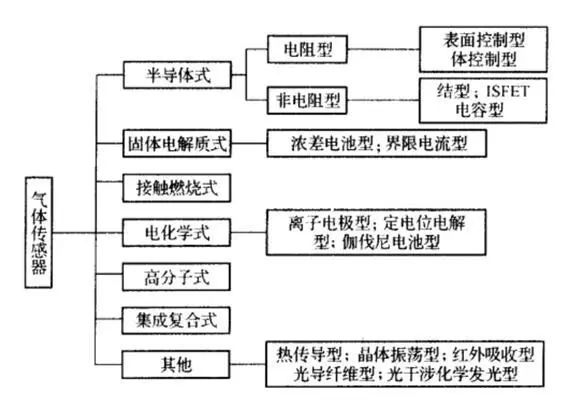
Semiconductor Gas Sensors
Semiconductor gas sensors can be divided into resistive and non-resistive types (junction type, MOSFET type, capacitive type). The principle of resistive gas-sensitive devices is that gas molecules cause changes in the resistance of sensitive materials; non-resistive gas-sensitive devices mainly include M()s diodes, junction diodes, and field-effect transistors (M()SFET), which utilize the principle that sensitive gases change the MOSFET’s gate voltage, similar to the principle structure of ISFET ion-sensitive sensor devices.
Resistive Semiconductor Gas Sensors
Operating Principle
It has been found that materials such as SnO2, ZnO, Fe2O3, Cr2O3, MgO, and NiO2 exhibit gas-sensitive effects. Gas-sensitive thin films made from these metal oxides are impedance devices, where gas molecules can exchange ions with the sensitive film, leading to a reduction reaction that causes changes in the resistance of the sensitive film. As sensors, it is also required that this reaction must be reversible, meaning that to eliminate gas molecules, an oxidation reaction must also occur. The heater inside the sensor aids the oxidation reaction process. SnO2 thin-film gas-sensitive devices are currently mainstream products due to their good stability, ability to work at lower temperatures, detection of multiple gas types, and mature technology. Additionally, Fe2O3 is also a widely used and researched material. Besides the traditional three categories of SnO, SnO2, and Fe2O3, a number of new materials have been developed, including single metal oxide materials, composite metal oxide materials, and mixed metal oxide materials. The research and development of these new materials have significantly improved the characteristics and application range of gas sensors.
Selective sensitivity is a key performance of gas sensors. For instance, SnO2 thin films are sensitive to various gases, and improving the selectivity and sensitivity of SnO2 gas-sensitive devices has always been a research focus. The main measures include: adding different noble metals or metal oxide catalysts to the substrate material, setting appropriate operating temperatures, and using filtering devices or permeable membranes to filter sensitive gases externally. Doping SnO2 materials is a primary method to improve sensor selectivity; adding noble metals such as Pt, Pd, and Ir can effectively enhance the sensitivity and response time of the components, and different catalysts lead to different adsorption tendencies, thus improving selectivity. For example, doping noble metals Pt, Pd, and Au in SnO2 gas-sensitive materials can improve sensitivity to CH4, while doping Ir can reduce sensitivity to CH4, doping Pt and Au can enhance sensitivity to H2, and doping Pd can reduce sensitivity to H2.
The operating temperature affects the sensitivity of the sensor. The left image below shows the resistance characteristics curve of SnO2 gas-sensitive devices at various gas temperatures. As seen from the graph, the sensitivity of the device to various gases varies at different temperatures, and this characteristic can be utilized to identify gas types.
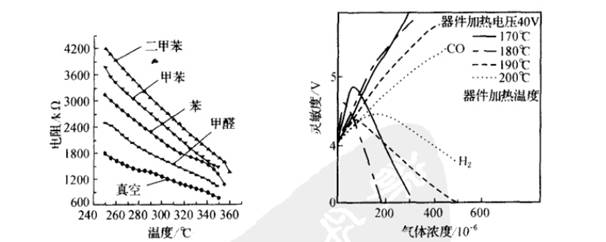
The preparation process also significantly impacts the gas-sensitive characteristics of SnO2. For instance, adding ThO2 to SnO2 and altering the sintering and heating temperatures can produce different gas-sensitive effects. By adding 3-5% ThO2 and 5% Sm2 to SnO2, sintering at 600°C in an H2 atmosphere to create thick film devices that operate at 400°C can serve as CO detection devices. The right image above shows the characteristics of gas-sensitive devices sintered at 600°C. It is evident that within the operating temperature range of 170-200°C, the sensitivity curve to H2 is parabolic, while changing the operating temperature has little effect on CO, thus utilizing this characteristic can detect H2. However, devices sintered at 400°C operating at 200°C show similar linear shapes for sensitivity curves to both H2 and CO, but the sensitivity to CO is significantly higher, allowing for the creation of CO-sensitive gas sensors.
Structure and Parameters
SnO2 resistive gas-sensitive devices typically use sintering processes. They are made from porous SnO2 ceramics as the substrate material, with the addition of various other substances, and are sintered using ceramic manufacturing techniques, embedding heating resistors and measurement electrodes during sintering. Additionally, thin film devices and multilayer film devices made using evaporation and sputtering techniques also exhibit high sensitivity and good dynamic characteristics. There are also thick film devices and mixed film devices made using screen printing techniques, which have high integration, easy assembly, user-friendly operation, and are conducive to mass production.
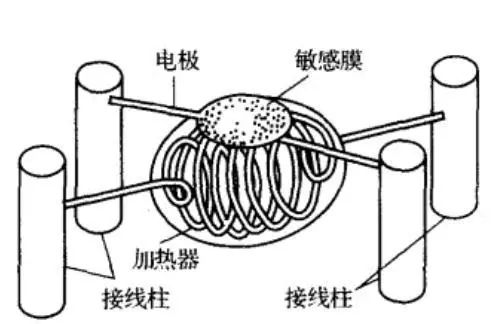
The image below shows a typical structure of a resistive gas sensor, which mainly consists of SnO2 sensitive elements, heaters, electrode leads, bases, and stainless steel mesh covers. This sensor features a simple structure, is easy to use, and can detect reducing gases, flammable gases, vapors, etc.
The main characteristic parameters of resistive gas sensors are:
1. Inherent resistance R0 and working resistance Rs
The inherent resistance Ro, also known as normal resistance, indicates the resistance value of the gas sensor under normal air conditions. The working resistance Rs indicates the resistance value of the gas sensor in a specific concentration of the measured gas.
2. Sensitivity S
Usually expressed as S=Rs/R0, sometimes also represented by the ratio of component resistance values in two different concentrations C1 and C2: S=Rs(C2)/R0(C1).
3. Response time T1
This reflects the dynamic characteristics of the sensor, defined as the time required for the sensor’s resistance to stabilize at a certain concentration of gas after contact. It is often expressed as the time to reach 63% of the change rate of the resistance value at that concentration.
4. Recovery time T2
Also known as desorption time, this reflects the dynamic characteristics of the sensor, defined as the time required for the sensor’s resistance value to return to its normal air condition value after detaching from the detected gas.
5. Heating resistance RH and heating power PH
RH is the resistance value of the heating wire providing the working temperature for the sensor, while PH is the heating power required to maintain normal working temperature.
Resistive gas sensors have advantages such as low cost, simple manufacturing, high sensitivity, fast response speed, long lifespan, low sensitivity to humidity, and simple circuitry. However, they also have disadvantages, such as the necessity to operate at high temperatures, poor selectivity to gases, dispersed component parameters, unsatisfactory stability, and high power requirements. When detecting gases containing sulfides, they are prone to poisoning.
Non-resistive Semiconductor Gas Sensors
Non-resistive types are also a common category of semiconductor gas-sensitive devices that are easy to use, do not require setting operating temperatures, and are suitable for integration, thus finding widespread application. The main types include junction type and MOSFET type.
Junction Gas Sensitive Devices
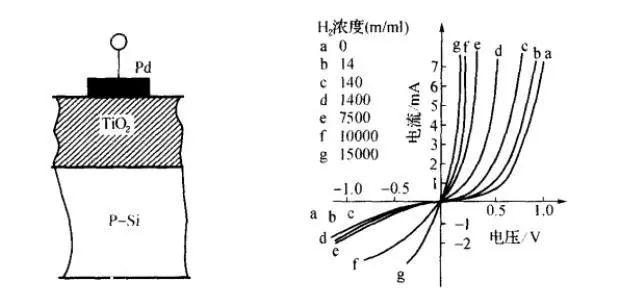
Junction gas-sensitive sensors, also known as gas-sensitive diodes, work by utilizing the gas to change the rectifying characteristics of the diode. Its structure is shown in the left image below. The principle is that noble metal Pd selectively reacts with hydrogen gas, forming a contact barrier with the semiconductor. When a forward bias is applied to the diode, electrons flow from the semiconductor to the metal, thus increasing conduction. When a reverse bias is applied, there is virtually no change in the carriers, which is the rectifying characteristic of Schottky diodes. In the detection atmosphere, due to the adsorption of hydrogen gas, the work function of the noble metal changes, weakening the contact barrier, leading to an increase in carriers and thus increasing the forward current, causing the rectifying characteristic curve of the diode to shift left. The right image below shows the characteristic curve of a Pd–TiO2 gas-sensitive diode in air at different concentrations of H2. Therefore, by measuring the forward current of the diode, the concentration of hydrogen gas can be detected.
MOSFET Gas Sensitive Devices
The left shift of the characteristic curve of the gas-sensitive diode can be viewed as a change in the conduction voltage of the diode, and if this occurs at the gate of a field-effect transistor, it will change the threshold voltage UT of the field-effect transistor. Using this principle, MOSFET gas-sensitive devices can be fabricated.
Hydrogen-sensitive MOSFETs are the most typical gas-sensitive devices, made with a palladium (Pd) gate. In an atmosphere containing hydrogen gas, due to the catalytic effect of palladium, hydrogen molecules decompose into hydrogen atoms that diffuse to the interface between palladium and silicon dioxide, ultimately causing a change in the threshold voltage UT of the MOSFET. During use, the gate and drain are often shorted to ensure that the MOSFET operates in the saturation region, where the drain current ID=β(UGS—UT)², and this circuit can be used to measure the concentration of hydrogen gas.
Characteristics of Hydrogen-sensitive MOSFETs:
1. Sensitivity
When the hydrogen gas concentration is low, hydrogen-sensitive MOSFETs exhibit very high sensitivity; a change of 1ppm in hydrogen gas concentration can yield a value of △UT up to 10mV. However, when the hydrogen gas concentration is high, the sensor’s sensitivity decreases.
2. Gas Selectivity
The “gaps” between palladium atoms allow hydrogen atoms to pass through, thus the palladium gate only allows hydrogen gas to pass, providing excellent selectivity.
3. Response Time
The response time of this device is influenced by temperature and hydrogen gas concentration; generally, higher temperatures and higher hydrogen concentrations lead to faster responses, with response times at room temperature being on the order of tens of seconds.
4. Stability
In practical applications, the threshold voltage UT exhibits a drift over time. To address this, a layer of SiO2 insulating layer is grown in an HCl atmosphere, which can significantly improve the drift of UT.
Aside from hydrogen, other gases cannot pass through the palladium gate. To create Pd–MOSFET gas-sensitive sensors for other gases, specific measures must be taken. For instance, to create CO-sensitive MOSFETs, small holes of about 20nm must be made on the palladium gate to allow CO gas to pass through. Additionally, since Pd–MOSFETs exhibit high sensitivity to hydrogen but low sensitivity to CO, a protective layer of aluminum about 20nm thick can be evaporated on the palladium gate to prevent hydrogen from passing through. Palladium has a weak catalytic effect on ammonia decomposition, so a layer of active metals such as Pt, Ir, or La must first be deposited on the SiO2 insulating layer before fabricating the palladium gate to create an ammonia-sensitive MOSFET.
Solid Electrolyte Gas Sensors
Solid electrolytes are solid materials that exhibit ionic conductivity characteristics similar to those of electrolyte solutions. When used as gas sensors, they function as batteries. They do not require gas to dissolve in an electrolyte through a permeable membrane, thus avoiding issues like solution evaporation and electrode consumption. Due to their high conductivity, good sensitivity, and selectivity, these sensors have been widely used in various fields such as petrochemicals, environmental protection, mining, and food industries, second only to metal-oxide semiconductor gas sensors in importance.
Principle of Solid Electrolyte Oxygen Sensors
Solid electrolytes exhibit significant conductivity only at high temperatures. Zirconia (ZrO2) is a typical material for gas sensors. Pure zirconia has a monoclinic crystal structure at room temperature, and when the temperature rises to around 1000°C, it undergoes a polymorphic transformation from monoclinic to cubic structure, accompanied by volume shrinkage and an endothermic reaction, thus forming an unstable structure. By incorporating stabilizers such as calcium oxide (CaO) or yttrium oxide (Y2O3) into ZrO2, it can be stabilized into a stable cubic crystal structure, with the degree of stability depending on the concentration of the stabilizer. After adding stabilizers to ZrO2, it is sintered in an 1800°C atmosphere, during which some zirconium ions are replaced by calcium ions, forming (ZrO·CaO). Since Ca2+ is a divalent cation and Zr4+ is a tetravalent cation, to maintain electrical neutrality, oxygen ions O2- vacancies are generated within the crystal. This is the reason why (ZrO·CaO) is able to conduct oxygen ions at high temperatures, resulting in (ZrO·CaO) becoming an oxygen ion conductor in the range of 300-800°C. However, to truly transmit oxygen ions, there must be a difference in oxygen partial pressure on both sides of the solid electrolyte (oxygen gradient), forming what is known as a concentration battery. Its structural principle is shown in the image below, with porous noble metal electrodes on both sides, and a dense ZrO·CaO material in the middle forming a sandwich structure.
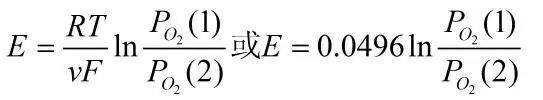
Let the oxygen partial pressures on both electrodes be PO2(1) and PO2(2), and the following reactions occur at the two electrodes:
(+) electrode: PO2(2), 2O2-→O2+4e
(-) electrode: PO1(1), O2+4e→2O2-
The electromotive force of the above reaction can be expressed using the Nernst equation:
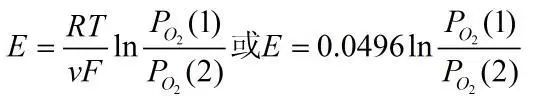
It can be seen that at a certain temperature, by fixing PO2(1), the concentration of the oxygen gas to be measured at the (+) electrode can be determined using the above equation.
Fixing PO2(1) essentially forms a fixed potential electrode at the (-) electrode, known as a reference electrode, which can be a gas reference electrode or a coexisting phase reference electrode. A gas reference electrode can be air or other mixed gases, such as H2–H2O, CO–CO2, which can also form a fixed PO2(1). A coexisting phase reference electrode refers to a mixture of metal-metal oxides or low-valent metal oxides-high-valent metal oxides (solid phase) that can react with oxygen (gas phase) to form a constant oxygen pressure, thus serving as a reference electrode.
In addition to measuring oxygen, solid electrolyte sensors made from β-Al2O3, carbonates, NASICON, etc. can also be used to measure gases like CO, SO2, NH4, etc. In recent years, gas sensors using antimony oxide, La3F, etc., which can operate at low temperatures, have emerged and can be used to detect cations.
Infrared Gas Sensors
Operating Principle
Molecules composed of different atoms have unique vibrational and rotational frequencies. When exposed to infrared light of the same frequency, they undergo infrared absorption, causing changes in infrared light intensity. By measuring these changes, the gas concentration can be determined. It should be noted that vibration and rotation are two different forms of motion, and each corresponds to different infrared absorption peaks. Therefore, generally, a single gas molecule will have multiple infrared absorption peaks. Determining the gas type accurately requires examining all absorption peaks in the mid-infrared range, i.e., the gas’s infrared absorption fingerprint. However, under known environmental conditions, a rough determination of gas type can be made based on the position of a single infrared absorption peak. Since all substances above absolute zero (−273°C) emit infrared radiation, and infrared radiation is positively correlated with temperature, infrared gas sensors are typically composed of a pair of infrared detectors to eliminate variations caused by environmental temperature changes.
A complete infrared gas sensor consists of an infrared light source, optical cavity, infrared detector, and signal conditioning circuit.
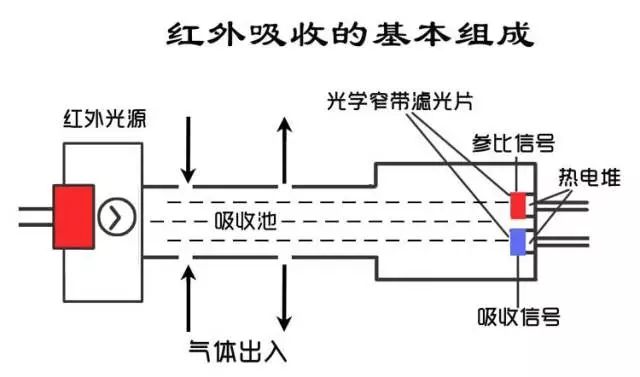
Why Can’t Infrared Gas Sensors Measure Gases Composed of the Same Atoms, Such as Oxygen, Hydrogen, and Nitrogen?
The moon and the earth, the earth and the sun are connected by gravitational force. Molecules are bonded together by chemical bonds. If both were ideal spheres and there were no other gravitational interferences, the orbit of the earth would be circular; in reality, neither condition holds, and thus the orbit is elliptical, meaning that the distance between the earth and the sun varies continuously between a short radius and a long radius, indicating vibration, with a cycle lasting an entire year. During this process, when the earth is at the short radius point and the long radius point, the gravitational force between it and the sun is different, indicating different energy levels. In molecules, the connection between atoms relies on chemical bonds, and the spatial distance, angle, and orientation between atoms constantly change due to uneven electron distribution, indicating vibration and rotation. Different molecules have unique vibrational and rotational frequencies; when exposed to infrared light of the same frequency, resonance occurs, causing changes in the distance between atoms and electron distribution, which leads to changes in dipole moments, resulting in infrared absorption (the same applies to ultraviolet absorption).
The above content includes two basic conditions for infrared absorption: resonance and changes in dipole moments. Both conditions must be met for infrared absorption to occur.
Why do molecules composed of the same atoms, such as oxygen, hydrogen, and nitrogen, lack infrared absorption peaks? The first condition is that the vibrational frequency of the gas molecule must match the frequency of the incident infrared light, while the second condition is that changes in dipole moments must occur. It’s easy to understand that the first condition can be met, but the second condition is impossible.
In molecules composed of the same atoms, the centers of positive and negative charges overlap completely, meaning that the dipole moment is zero. As a result, the electron distribution in the molecule is balanced. Given the low energy density characteristic of infrared light, its irradiation cannot alter this balance, nor can it ionize the molecule, meaning that energy changes cannot occur. In contrast, for molecules composed of different atoms, take the example of water (vapor) molecules, where the distribution of electrons is biased towards the oxygen end, meaning that microscopically, the hydrogen end of the water molecule is positively charged while the oxygen end is negatively charged, indicating that the centers of positive and negative charges do not overlap, resulting in a non-zero dipole moment. This is because oxygen has a stronger ability to attract electrons than hydrogen.
When exposed to infrared light with a frequency matching that of the vibration and rotation of water molecules, the electron distribution in the water molecule shifts further towards the oxygen end, reducing the average distance between hydrogen and oxygen, thus shortening the dipole moment and increasing energy levels. Therefore, when water molecules are exposed to infrared light, they transition from a low energy level to a high energy level, leading to infrared absorption. A simple way to understand this is that when infrared light encounters molecules composed of the same atoms, due to being ideal elastic spheres, their interaction is a perfectly elastic collision, involving only energy exchange with no energy transfer. In contrast, the interaction between molecules composed of different atoms and infrared light involves energy transfer. Thus, the principle of infrared absorption cannot be applied to molecules composed of the same atoms.
Non-dispersive Infrared Absorption Gas Sensors
Non-dispersive: white light passing through a prism is divided into seven colors (red, orange, yellow, green, blue, indigo, violet). This prism acts as a spectral separation system, capable of splitting seven colors of light. Optical systems with spectral separation systems are known as dispersive optical systems, while those without are called non-dispersive optical systems. Non-dispersive systems are simple, reliable, compact, and inexpensive. The white light, ultraviolet, and infrared light we perceive are mixtures of light with different frequencies and wavelengths, while monochromatic light is light of a single frequency and wavelength. As previously mentioned, infrared absorption occurs only when the frequency of infrared light matches the vibrational and rotational frequencies of gas molecules. In theory, when designing gas sensors, we hope to use monochromatic light to irradiate the gas or obtain monochromatic light using a grating (filter) after irradiation.
Non-dispersive infrared gas sensors typically consist of a light source, optical cavity, filter (grating), detector, and signal conditioning circuit, with the filter and detector being integrated within the sensor.
Advantages of Infrared Gas Sensors:
1. All gases can be measured except those composed of the same atoms.
2. Full range.
3. The sensing process does not interfere with the measurement.
Disadvantages:
1. Expensive. Infrared gas sensors essentially operate as temperature sensors where infrared radiation causes temperature changes in the detector, leading to changes in electrical performance. The sensing process is complex and requires the system to have the following characteristics: the light source must provide stable infrared radiation; the optical cavity must have stable physical and chemical properties; the filter and infrared detector must be stable. These issues can be reasonably addressed through proper manufacturing techniques, but the high manufacturing costs lead to high prices.
2. In ordinary designs using broad-spectrum infrared light sources, filters, and detectors, the filters themselves cannot achieve ideal selective filtering, thus interference, especially from water, is always present. The deep-rooted reason for selectivity issues is that many different gas molecules share similar chemical bonds, leading to overlapping infrared absorption.
3. Dust, background radiation, and strong adsorption of gases, liquids, and solids can all affect the detection results.
Catalytic Combustion Gas Sensors
Operating Principle
Generally composed of high-purity platinum wire coils with diameters of 15um, 20um, or 30um, wrapped in a spherical catalyst carrier. Under certain temperature conditions, when combustible gases contact the catalyst, a vigorous non-flame combustion reaction occurs with the adsorbed oxygen on the surface, releasing heat that causes temperature changes in the platinum wire coil. The change in temperature leads to changes in the resistance of the platinum wire coil, allowing the measurement of gas concentration through resistance changes.
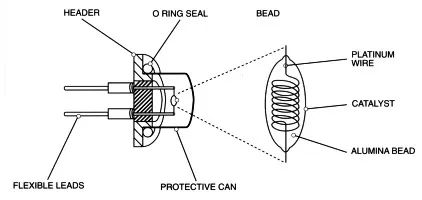
Thus, rather than saying the catalytic element is a gas sensor, it is more accurate to describe it as a temperature sensor. To mitigate interference from environmental temperature variations, the catalytic element is typically paired to form a complete component. One of the pair responds to the gas, while the other only reacts to environmental temperature, allowing the two elements to offset each other and eliminate the effects of environmental temperature changes.
Compared to semiconductor elements, the sensing process in catalytic elements is more complex. In the former, the chemical reaction between the gas and the sensor directly leads to changes in the sensor’s resistance and thus electrical signals. In contrast, in catalytic elements, the chemical reaction occurring on the catalyst first causes temperature changes on the surface and within the carrier, and this temperature change, through thermal transfer, ultimately leads to changes in the resistance of the platinum wire coil, completing the entire sensing process.
Existing Issues
The complexity of the sensing process increases the likelihood of issues arising.
1. For long-chain organic compounds and unsaturated hydrocarbons, incomplete reactions lead to carbon deposits that affect the reaction process without significantly impacting electron transfer in semiconductor sensors. However, in catalytic sensors, the presence of carbon not only affects the reaction process but also severely impacts heat transfer, resulting in reduced efficiency in transferring the heat generated by the reaction to the sensor. Consequently, with the same gas concentration and heat released, the presence of carbon leads to only minimal temperature changes in the sensor, thus reducing sensitivity.
2. Due to the need for heat transfer, the reaction must be completed instantaneously to ensure high reaction efficiency, necessitating a large amount of nanoscale catalysts and nanoscale pores. These characteristics are conducive to sensing but also increase the risk of poisoning.
3. The linearity of catalytic elements is determined by two factors: a. The temperature sensing material’s (platinum wire) resistance-temperature characteristics are linear. b. The heat released during reactions below the explosive limit is linear with gas concentration. Therefore, any change in either factor will lead to changes in sensor linearity. In practice, the platinum wire will continuously sublime and thin, increasing resistance; the linear relationship between the heat released and concentration only holds when gas concentrations are below the explosive limit.
Future Development
The future of catalytic elements primarily depends on advancements in processing technology:
1. Structural improvements to address drift caused by vibrations.
2. Improvements to filtering layers to address poisoning issues.
3. Development of new materials to reduce carbon buildup.
4. Ensuring manufacturing processes support design implementation, such as avoiding deformation.
5. MEMS integration. It should be noted that improvements in device structure, packaging, and manufacturing processes will not only enhance the overall performance of the components but also lead to new applications. Compared to semiconductors, the challenge of MEMS integration for catalytic elements lies in achieving higher catalytic efficiency and thermal efficiency on smaller surface areas.
6. The application positioning of catalytic elements will become more precise and specialized.
7. Catalytic elements will not be eliminated.
Electrochemical Sensors
Electrochemistry is the study of the relationship between electrical and chemical behavior. The most important applications of this field are efficient conversion between electrical energy and chemical energy and high power density storage technology. Essentially, sensors are energy conversion devices; for instance, pressure sensors convert mechanical energy into electrical energy. Therefore, it is easy to understand that electrochemical gas sensors function as batteries, known as gas fuel cells.

The most common battery consists of a collection of conductive chemicals enclosed within two electrodes made of different materials, connected by wires to generate electricity. For example, in a lead-acid battery, sulfuric acid solution serves as the conductive chemical. When lead is placed in it, electricity is generated at the interface where lead and sulfuric acid meet. Adding lead oxide into the mix also generates electricity at its interface. The two interfaces have different charges, creating a voltage that allows electrons to flow from lead to lead oxide, converting lead into lead oxide and lead oxide into lead sulfate. The quantity of electric charge is correlated with the chemical quantity and reaction process.
The most important concepts here are: first, inserting a conductor into a conductive chemical creates an electric potential at the interface; second, inserting different conductors into the same substance generates different electric potentials. Third, the conductive circuit consists of both the battery and external wires. The movement of electrons occurs in the wires, while ions move within the battery. Thus, the conduction process is accomplished through the movement of both electrons and ions.
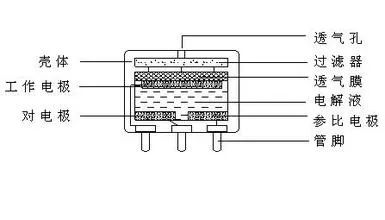
The electrochemical CO gas sensor is a chemical battery, specifically a CO fuel cell. In this cell, CO acts as the electron provider (working electrode), while oxygen serves as the electron receiver (counter electrode), with sulfuric acid solution as the electrolyte. The primary difference from lead-acid batteries lies in the electrode materials; in electrochemical gas sensors, the electrodes are gases, while in lead-acid batteries, they are solids. The electrodes in electrochemical gas sensors are known as gas electrodes. In the electrochemical CO gas sensor, the working electrode CO serves as the provider of electrons, and the process of CO providing electrons cannot occur without contact with sulfuric acid solution. This process requires conditions that lower the difficulty of CO providing electrons, typically provided by porous platinum electrodes (or other electrocatalytic conductive electrodes). The electrons provided by CO need to be collected and conducted, also facilitated by the porous platinum electrode.
Similarly, the oxygen electrode, acting as the counter electrode, also requires assistance from a porous platinum electrode to obtain electrons. The platinum electrode serves as the reaction platform. Although the principle of electrochemical sensors is straightforward, achieving reliable and precise sensing is challenging: first, the platinum electrode must maintain a stable porous structure, with sufficient pore quantity, allowing sulfuric acid solution to enter the pores, and CO (or oxygen) must also access the pores, where the three-phase interface (gas (CO) – solid (Pt) – liquid (sulfuric acid solution)) completes the electron provision. Thus, how to maintain a stable three-phase interface under long-term immersion in sulfuric acid, impacts of electrochemical reactions, and electrophoresis, is central to achieving reliable and precise sensing. Second, the sulfuric acid solution must remain stable, non-volatile, non-hygroscopic, and leak-proof. Any change in the quality of the sulfuric acid solution will lead to pressure changes within the sensor, resulting in variations in the three-phase interface. Third, the contact stress between the electrode and the sulfuric acid solution, dictated by packaging and material physical properties, must remain stable and unchanging.
Currently, the primary issues with electrochemical sensors stem from these factors. One of the core technologies and processes for electrochemical sensors is how to construct a physically reasonable and reliable stable pore structure in the electrodes, which is closely related to sensitivity, response recovery, lifespan, and temperature characteristics. Secondly, packaging is crucial. Issues with electrochemical sensors include loss of activity due to dehydration under dry conditions, leakage under high humidity, deactivation due to prolonged exposure to the measured gas, and loss of activity due to the disintegration of the electrode pore structure. Performance issues manifest as leakage, short lifespan (compared to other principles), and larger size. In terms of manufacturing, this complexity is reflected in design, processes, and high manufacturing costs.
The future of electrochemical sensors is clear: the direction involves solidifying the electrolyte at room temperature and achieving MEMS integration based on this. Solid-state and MEMS-integrated electrochemical sensors can not only overcome most manufacturing-related issues but also inspire new applications, leading to new growth for enterprises. The electrochemical sensors of the future will be highly integrated, easily assembled, and compact electronic systems. However, this outcome still cannot overcome performance changes caused by prolonged contact with high concentrations or the measured gas.
PID – Photoionization Detectors
PID consists of a UV light source and a gas chamber. The UV emission principle is similar to that of fluorescent lamps, only at a higher frequency and energy. When the gas to be measured enters the gas chamber, the ultraviolet light emitted by the UV lamp ionizes it, generating a flow of charge, which is directly proportional to the gas concentration. Measuring the charge flow allows the determination of gas concentration.
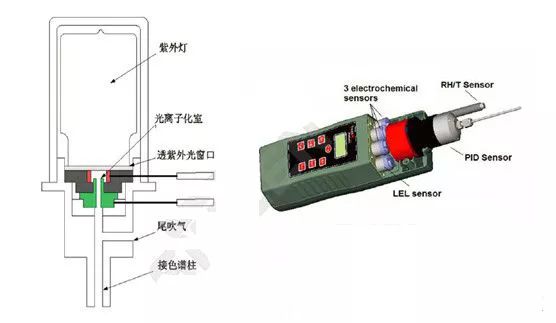
Special Gases: Physical states vary, and the chemical processes and reaction products are complex and diverse. This includes inorganic gases such as ammonia and organic gases such as toluene.
The various gas sensors introduced earlier face significant challenges when detecting complex gases. For instance, in detecting organic vapors, the infrared absorption principle encounters insurmountable difficulties: a. Due to their large molecular weights, organic vapors have longer characteristic absorption wavelengths, resulting in low sensitivity due to minimal energy changes upon infrared absorption.b. Long-chain organic vapors are prone to adsorption, adhering to detectors and disrupting light transmission.c. It is impossible to achieve total detection of VOCs. For infrared systems to achieve total evaluation, filters, detectors, and broadband infrared light sources that respond across the entire spectrum are required. Such requirements are not only difficult to realize but, even if achieved, inorganic gases and water would naturally interfere across the entire spectrum. In chemical sensors, semiconductors are easily interfered with by inorganic gases, temperature, and humidity, leading to drift and low concentration resolution. Although their detection range is wide and covers many gas types, they are still only suitable for low-end applications. In this context, PID is a better choice for VOC detection in industrial settings.
Compared to other sensors, the most notable feature of PID is its sensitivity to very few inorganic gases, such as ammonia and phosphine. This is because most inorganic gases have high ionization energies (greater than 11.7eV). Currently, the highest energy output from PID lamps is only 11.7eV. Therefore, in petrochemical parks, the response of PID can be considered a response to VOCs.
Working Principle of PID
1. High-purity rare gases such as argon and krypton are filled into a vacuum glass chamber.
2. The glass chamber is sealed with a magnesium fluoride single crystal window, which is transparent to ultraviolet light.
3. Electrodes are placed on the outer wall of the glass chamber.
4. An electric field is applied to the magnesium fluoride window and electrodes, creating a gas chamber for the measured gas. This forms a complete ultraviolet lamp capable of ionizing VOCs. During operation, a high-frequency electric field is applied to the outer glass chamber, ionizing the rare gases within the UV lamp into electrons and ions. When electrons and ions recombine, they emit energy in the form of ultraviolet light. The ultraviolet light passes through the magnesium fluoride window to reach the gas chamber, where the measured gas is ionized by the ultraviolet light, generating electrons and ions. The charge generates a current under the electric field, allowing measurement.
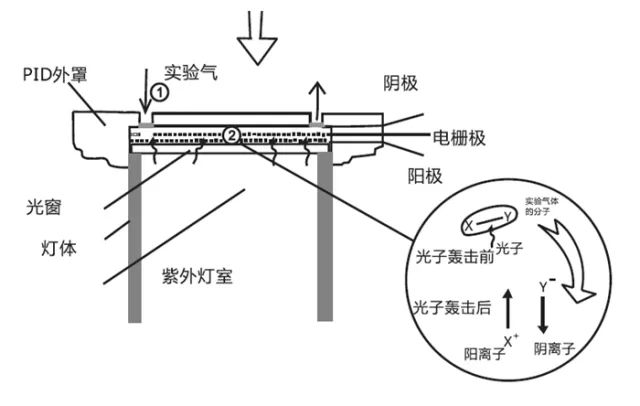
Stable Operation of PID Requires:
1. PID must emit sufficient energy to ionize the measured gas;
2. The high-frequency electric field generating ultraviolet light must be stable.
3. The glass chamber must be free of impurities, as these can cause additional ionization and affect UV emission efficiency.
4. The UV spectrum must be stable and uniform.
5. The transmission of UV light to the gas chamber must be stable and uniform, without interaction with the metal electrode materials constituting the gas chamber, which could lead to heavy metal deposits that block UV light from reaching the chamber.
These requirements dictate that the light-emitting gas must be a gas to ensure uniform emission and transmission. The chamber must not contain impurities to prevent additional ionization. These stringent conditions ultimately determine the limitations of PID applications.
Why Current PID Cannot Measure Propane, Ethane, Methane, and Most Inorganic Substances
The essence of PID is to measure the charge flow after ionizing the measured substance, which requires energy. The most common energy output from PID UV lamps is 8.3eV, 9.8eV, and 10.6eV. However, the energy required to ionize methane is 12.6eV, for ethane it is 11.56eV, for propane it is 10.95eV, and for carbon dioxide, it is 13eV. In fact, there is a strong desire to develop PID lamps that emit higher energy, but the limitations stem from the limited types of rare gases and the fact that the UV wavelength (energy) is determined by the electronic energy levels of the rare gases themselves, which cannot be altered. Another limiting factor is the transparency of window materials to specific wavelengths of UV light, which depends on the lattice constants of the window materials, and options are currently very limited. Although efforts have been made to develop 11.7eV light sources, the only suitable window material is lithium fluoride (LiF), which is highly hygroscopic, resulting in a lifespan of only two months for 11.7eV PID lamps. Thus, due to the output energy limitations, current UV lamps still cannot detect substances like methane that have higher ionization energies.
Why PID Lacks Selectivity?
If the chosen PID emits UV radiation at 10.6eV, it means that all gas molecules in the environment with ionization energies less than 10.6eV will be ionized. The charge flow measured is the sum of the charge flows from all ionized gases, rather than from a specific gas. Thus, PID’s lack of selectivity is determined by this.
During PID operation, when the substances ionized in the gas chamber recombine, long-chain molecules, dust, and other particles may deposit on the window surface. Additionally, the ion flow generated during sensor operation can also lead to heavy metal deposits on the window surface, which clearly affects UV transmission, resulting in zero drift and reduced sensitivity, impacting detection results. In fact, in addition to the preparation technology of the PID lamp and gas chamber design, the cleaning technology for the UV light transmission window is also a core technology.
Future of PID
1. PID will always exist as an ideal non-radioactive ion source;
2. Improve the vacuum level before filling PID lamps and the purity of the filling gas to enhance light emission efficiency and stability;
3. Develop new window materials and processing precision to improve light transmission, output uniformity, packaging quality, and stability and longevity;
4. Prevent dispersion from causing heavy metal deposits on the window to extend lifespan;
5. Develop window cleaning technologies to prevent deposits from large organic molecules and small particles;
6. Develop higher energy output, long-life PID lamps;
7. Smaller size.
Future Directions for Gas Sensors
The research on gas sensors covers a wide range and is complex, belonging to interdisciplinary studies. To effectively enhance the performance indicators of sensors, collaboration among researchers from multiple disciplines and fields is essential. The development of gas-sensitive materials and the rational design of sensor structures based on different principles have always been a focus for researchers. The future development of gas sensors will also revolve around these two aspects. Specific manifestations include:
Further development of gas-sensitive materials, on one hand, involves searching for new additives to enhance the performance of already developed gas-sensitive materials; on the other hand, fully utilizing new material preparation techniques such as nanomaterials and thin films to find superior gas-sensitive materials.
The development and design of new gas sensors will focus on creating new types of gas sensors based on the different effects that gases may have on gas-sensitive materials. In recent years, the successful development of new sensor types such as surface acoustic wave gas sensors, optical gas sensors, and quartz crystal oscillators has expanded the designers’ horizons. Biomimetic gas sensors are also under research.
Further research into the sensing mechanisms of gas sensors is necessary, as new gas-sensitive materials and new sensor types continue to emerge. Clarifying the mechanisms will help avoid detours in future work.
The rapid advancement of intelligent production and living conditions poses higher demands on gas sensors, making intelligence a necessary path for their development. Intelligent gas sensors will develop based on the comprehensive use of technologies from multiple disciplines, including micromechanics and microelectronics, computer technology, signal processing technology, circuit and system technology, sensing technology, neural network technology, and fuzzy theory.
The rapid development of biomimetic gas sensors, such as the dog’s nose, which is an ideal gas-sensitive sensor with excellent sensitivity and selectivity, will be one of the important directions in the development of gas sensors by integrating biomimicry and sensor technology to create an “electronic nose” similar to a dog’s nose.
Source: Sensor Technology
Editor: Song Bingjia
