Written by | Yang Ben (Northwestern University, USA)
Editor | Qi

Movement is one of the most fundamental functions of the brain. Adapting, learning, and initiating appropriate movements in different environments are crucial for survival. The structure known as the basal ganglia is primarily responsible for movement regulation and learning, consisting of several nuclei. The entry nucleus of the basal ganglia is called the striatum, which mainly receives sensory signals from the cortex and thalamus, and integrates dopamine signals from the midbrain to influence behavioral decisions. Dysfunction of the basal ganglia is associated with various movement disorders, such as Parkinson’s disease, Huntington’s disease, and Tourette’s syndrome.
After years of research by scientists in the field of basal ganglia, a basic understanding of the internal signal processing of the basal ganglia has been established. The classic Go/NoGo model indicates that a direct pathway promotes movement (Go), while an indirect pathway inhibits movement (NoGo). The overall functional structure of the basal ganglia resembles a funnel, processing sensory signals received by the striatum and sending them back to the cortex and directly to the brainstem, thereby regulating movement and motor learning【1, 2】. Mahlon DeLong and Alim Louis Benabid shared the 2014 Lasker Award for their contributions to the classic model and received the Breakthrough Prize in Life Sciences in 2014 and 2015, respectively. However, the classic model is overly simplified, and recent research indicates that details still need to be revised【3】.
So how is the signal transmitted from the cortex to the striatum? Research by Ann Graybiel and others over the years has indicated that signals from one area of the cortex project to different areas of the striatum, which then converge at the output of the basal ganglia【4】. Different regions of the striatum act like different experts, processing various signals. The latest research by rising star Arif Hamid in the field of basal ganglia regarding dopamine signals (the article is currently under review at Nature) supports the existence of different experts in different regions of the striatum, processing different cortical signals【5】. The Allen Brain Institute’s brain connectivity map (https://connectivity.brain-map.org/), the brain connectome project led by Chinese scholar Hongwei Dong at USC (now moved to UCLA) (https://cic.ini.usc.edu/), and previous research by Tianyi Mao at the Vollum Institute all indicate that there are topographic connections from the cortex to the striatum【6】. However, due to technical limitations, there is still a lack of comprehensive functional connectivity records between the cortex and striatum. Some current functional studies are limited to local connections and indicate that motor learning can enhance synaptic plasticity of local connections related to learning【7, 8】.
Recently, Kenneth Harris and Matteo Carandini from University College London, leading the CortexLab, published an article titled “Striatal Activity Topographically Reflects Cortical Activity” in Nature (with Dr. Andrew Peters as the first and corresponding author).This study combines the latest wide-field fluorescence imaging of the cortex and Neuropixel probe technology to simultaneously record neural calcium signals across the entire cortex and neural electrical signals spanning the entire striatum, confirming for the first time that striatal activity topographically reflects cortical activity patterns.

To record the entire cortical activity, the researchers used CaMK2a-tTA; tetO-GCaMP6s mice, where the CaMK2a promoter expresses the tetracycline-inducible GCaMP6s calcium fluorescent indicator in cortical excitatory neurons. To confirm that the calcium signals recorded by wide-field imaging of the cortex truly reflect neuronal activity, in some mice, the researchers inserted a Neuropixel probe into the striatum and also into the anteromedial visual cortex (VisAM) to record neuronal activity. After processing the cortical calcium signals with a deconvolution kernel, it was confirmed that they accurately reflected the cortical neural electrical signals, validating the reliability of this method.
Andrew Peters stated that the main difficulty of this project was to integrate wide-field imaging of the cortex, striatal Neuropixel electrodes, and behavior. If any one of these three experiments had a slight issue, the entire experiment would have to start over. The most challenging experiment was inserting the second Neuropixel electrode into the visual cortex, which added another risk.
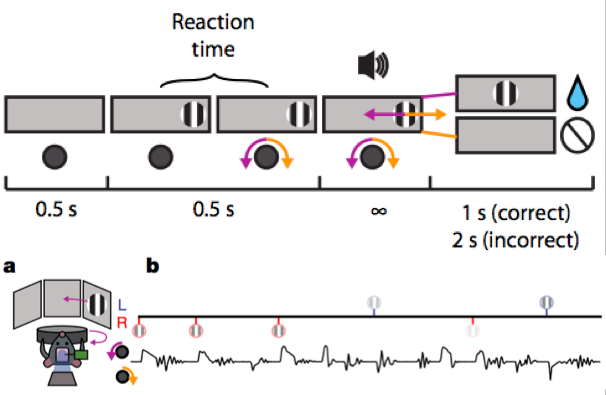
To differentiate the encoding of visual, motor, and reward information, the researchers designed a behavior as shown in the figure below. In front of the mouse, there is a visual screen and a wheel. In the first half second, there is no visual stimulus on the screen; after half a second, a striped image appears at one end of the screen, and the mouse can either turn the wheel or not, but turning the wheel does not change the position of the image on the screen; after another half second, an auditory stimulus prompts the mouse to turn the wheel to change the position of the image, where clockwise rotation moves the image to the right and counterclockwise rotation moves it to the left. If the image moves to the center of the screen, the mouse receives a reward of water, and after one second, the second round of training begins; if the image is moved off the screen, a white noise sound occurs, and after two seconds, a new training round starts without a reward for the mouse.
The researchers found that when the visual stimulus appeared, cortical activity mainly occurred in the visual cortex and anterior mid-cingulate cortex, while striatal activity was primarily in the dorsomedial striatum (DMS); when the wheel began to turn, cortical activity shifted forward and concentrated mainly in the limb-related somatosensory cortex (SSp, including upper limb, SSp-ul, and lower limb, SSp-ll), posterior primary motor cortex (posterior MOp), and medial secondary motor cortex (medial MOs), while striatal activity shifted outward to the dorsocentral striatum (DCS); when the mouse received the water reward, cortical activity shifted forward again and mainly concentrated in the orofacial somatosensory cortex (orofacial SSp, including mouth drinking, SSp-m), lateral primary motor cortex (lateral MOp), and lateral secondary motor cortex (lateral MOs), while striatal activity shifted outward to the dorsolateral striatum (DLS). Overall, cortical activity shifted from back to front, while striatal activity shifted from inside to outside, with both activities showing a topographic imaging relationship.
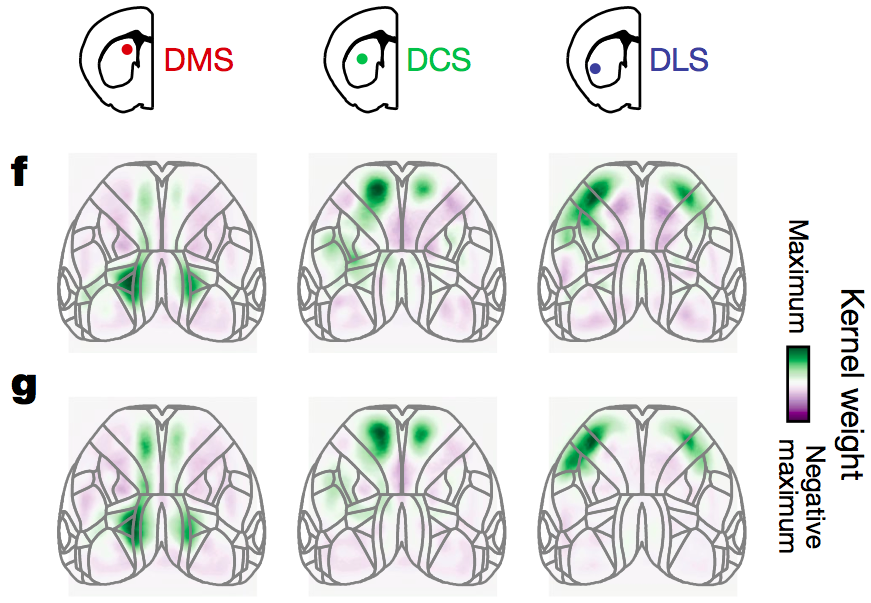
Interestingly, when the researchers compared the cortical and striatal activity of the mice when not performing tasks (visual noise stimulus), they found that both also exhibited a topographic imaging relationship, suggesting that the topographic imaging relationship between striatal and cortical activity is not influenced by the motor learning task (with f representing task execution and g representing non-task execution in the figure below).
Andrew Peters stated that based on the research of Ann Graybiel and others, he expected that there would be some degree of transformation of signals from the cortex to the striatum, or that there would be some different correspondence with behavior, or that there might be some striatal activity that could not be explained by cortical activity. Throughout the research process, they were looking for differences, but ultimately found consistency. This might be the most unexpected finding.
To prove that striatal activity indeed originates from cortical signals and is not merely correlated, the researchers placed a gel foam soaked in muscimol (a GABAA receptor antagonist that specifically activates GABAA receptors to inhibit neuronal activity) above the visual cortex to suppress its activity. After suppressing the activity of the visual cortex, the dorsomedial striatum (DMS) activity related to visual stimuli was also suppressed, while motor-related and reward-related striatal activity remained unaffected, proving that striatal activity indeed originates from cortical activity.
The main projection cells in the striatum are spiny projection neurons (SPNs), which account for over 90% of striatal cells. The aforementioned direct and indirect pathways are projected by direct pathway spiny projection neurons (dSPNs or dMSNs) and indirect pathway spiny projection neurons (iSPNs or iMSNs). In addition, there are interneurons, mainly fast-spiking interneurons (FSIs, inferred to be parvalbumin PV positive interneurons based on physiological activity), tonic active neurons (TANs, which fire continuously at a relatively fixed frequency, inferred to be cholinergic interneurons based on physiological activity), and low-threshold spike interneurons (LTS, which mainly express neuropeptide Y, somatostatin, nitric oxide synthase, calretinin, etc.).
To understand the correlation between different striatal cell activities and cortical activity, the researchers classified the cells recorded by the Neuropixel electrodes into MSNs, FSIs, and TANs, and found that the activity of MSNs and FSIs was consistent with the cortex, while TANs were less consistent. Under visual stimuli, DMS and DCS showed synchronized burst firing followed by a short pause in firing patterns, while DCS and DLS showed similar patterns under reward stimuli. The activity of MSNs and FSIs was consistent with the cortex, while the activity of TANs was not, which is consistent with previous studies on the different sources of signal inputs to striatal cells. Previous research has shown that, except for TANs, other cells mainly receive signals from the cortex, while TANs receive more signals from the thalamus【9】. The activities of MSNs, FSIs, and the cortex were all correlated, while the activity of TANs was only correlated with itself and not with the activities of MSNs, FSIs, or the cortex.
Finally, the researchers questioned whether motor learning would affect the correlation between cortical and striatal activities. They found that after training, the activity of the visual cortex did not strengthen, but the activity of DMS did increase, indicating that the projection from the visual cortex to DMS may have undergone synaptic plasticity enhancement. The increase in DMS activity mainly occurred in MSNs and TANs, while FSIs’ activity remained unchanged, suggesting that FSIs’ regulation of MSNs’ activity may not be influenced by motor learning. This increase in activity due to motor learning reflects overall activity, and individual MSNs or dMSNs and iMSNs may exhibit differences. For example, a previous study found that enhancement only occurred in MSNs related to the frequency of learning stimuli【7】, while another study found that learning-specific enhancement of synaptic plasticity occurred from the cortex to dMSNs during the completion of serial actions【8】. Additionally, although DCS activity also showed slight enhancement, the corresponding cortical activity also increased, indicating that there should not have been any synaptic plasticity changes.
In summary, this study combines the latest wide-field calcium imaging of the cortex and Neuropixel electrode technology to simultaneously record the neural activity of the cortex and striatum in vivo, confirming for the first time that striatal activity topographically reflects cortical activity patterns. This correlation is not influenced by specific motor scenarios, but motor learning can specifically enhance this correlation. This correlation exists with MSNs and FSIs but not with TANs, possibly because TANs receive more signals from the thalamus in addition to cortical signals. Finally, the enhancement of correlation does not occur in FSIs, suggesting that FSIs’ regulation of MSNs’ activity may not be influenced by motor learning. Overall, this represents a significant technical challenge and an important breakthrough in biological function (the encoding of information transmission between the cortex and striatum). It is believed that more interesting research will follow, leading to a better understanding of the basal ganglia’s regulation of movement and learning, and aiding in the better treatment of movement-related diseases.
Original link:https://www.nature.com/articles/s41586-020-03166-8
Editor: Qi Jiang
Swipe up to view references
1. DeLong, M. R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285 (1990).
2. Albin, R. L., Young, A. B. & Penney, J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
3. Parker, J. G. et al. Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature 557, 177–182 (2018).
4. Graybiel, A. M. The Basal Ganglia and Chunking of Action Repertoires. Neurobiology of Learning and Memory 70, 119–136 (1998).
5. Hamid, A. A., Frank, M. J. & Moore, C. I. Dopamine waves as a mechanism for spatiotemporal credit assignment. BioRxiv 729640,
6. Hunnicutt, B. J. et al. A comprehensive excitatory input map of the striatum reveals novel functional organization. Elife 5, 1–32 (2016).
7. Xiong, Q., Znamenskiy, P. & Zador, A. M. Selective corticostriatal plasticity during acquisition of an auditory discrimination task. Nature 521, 348–351 (2015).
8. Rothwell, P. E. et al. Input- and Output-Specific Regulation of Serial Order Performance by Corticostriatal Circuits. Neuron 88, 345–356 (2015).
9. Johansson, Y. & Silberberg, G. The Functional Organization of Cortical and Thalamic Inputs onto Five Types of Striatal Neurons Is Determined by Source and Target Cell Identities. Cell Rep 30, 1178–1194.e3 (2020).
