The annual oncology feast—the American Society of Clinical Oncology (ASCO) annual meeting—was held both online and offline in Chicago from June 3 to 7, 2022. The ASCO annual meeting is a prestigious academic exchange event in the global oncology field, showcasing the latest clinical oncology research achievements and cancer treatment technologies. [ONCO Frontier] will continue to bring you important research related to the conference for your learning and reference.
Research Title
Abstract No. 6005: ROMAN: A Phase III Trial of Avasopasem Manganese (GC4419) for the Treatment of Severe Oral Mucositis (SOM) in Patients with Locally Advanced, Non-Metastatic Head and Neck Cancer (LAHNC) Receiving Chemoradiotherapy (CRT).
Research Background
Intensity-modulated radiotherapy (IMRT) combined with cisplatin is an established treatment regimen for LAHNC, but approximately 70% of patients experience SOM (WHO grade 3 or 4), limiting their ability to eat solids (grade 3) or liquids (grade 4), often requiring nutritional support via feeding tubes. The focus of management is on symptom relief and supportive care (Elad 2020). There are no US-approved drugs for reducing SOM in LAHNC. Radiation-induced superoxide bursts initiate the development of oral mucositis (OM) (Sonis 2004). Avasopasem (GC4419, AVA) is an investigational selective small molecule superoxide dismutase mimetic designed to convert superoxide into hydrogen peroxide, potentially protecting normal cells from radiation while making cancer cells more susceptible to radiation (Riley DP 2006, El-Mahdy 2020).
In a randomized, double-blind, Phase IIb trial, AVA reduced the duration and incidence of SOM caused by CRT in the LAHNC group compared to placebo (PBO) (Anderson 2019). This trial (NCT03689712) further evaluated the safety and efficacy of AVA in reducing SOM caused by CRT in oral (OC) or oropharyngeal (OP) LAHNC.
Research Methods
Double-blind, PBO-controlled trial; patients receiving 60-72 Gy IMRT (>50 Gy to ≥2 oral mucosal sites) plus cisplatin (weekly or q3 weeks) were randomized in a 3:2 ratio to receive AVA 90mg or PBO (administered via intravenous infusion 60 minutes prior to each RT fraction). Trained assessors evaluated WHO OM scales every two weeks during RT and then weekly for 2 weeks. The primary endpoint was the incidence of SOM (WHO grade 3 or 4) at the end of IMRT. Secondary endpoints included the duration of SOM within 2 weeks post-IMRT and the incidence of grade 4 OM prior to the end of IMRT.
Research Results
N=407 (241 AVA/166 PBO); median age 61 years; 86% male; 82% OP. A relative reduction in SOM incidence of 16% was observed (54% vs 64%; p=0.045), with a relative reduction in SOM duration of 56% (median, 8 days vs 18 days; p=0.002), showing statistical significance. The incidence of grade 4 was reduced by 27% (p=0.052). Improvements were observed in multiple secondary and exploratory endpoints (Table 1). The frequency of adverse events between treatment groups (all grades, 3+ grades, severe) was comparable, with no clear AVA-specific toxicity or increased cisplatin-related toxicity.
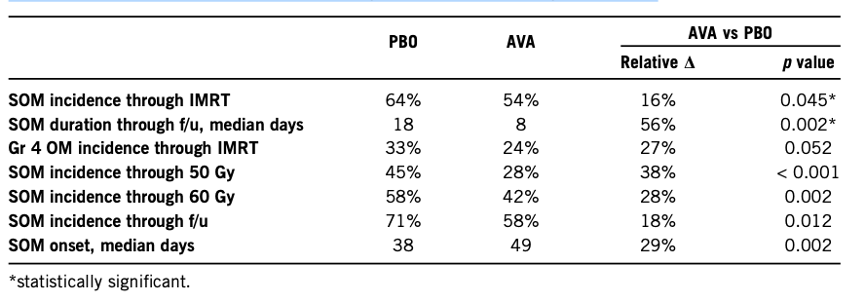
Table 1
Research Conclusion
Compared to PBO, AVA provided statistically significant and clinically meaningful improvement in SOM across multiple SOM metrics, with good tolerability, and the adverse event profile was consistent with expectations for IMRT/cisplatin in LAHNC. Clinical trial information: NCT03689712.
Visual Design | Huaxi, Meguro
Editor | Jingjing
Copyright Statement: This platform aims to help healthcare professionals better understand the latest developments in the relevant disease areas. The content published on this platform does not imply agreement with its descriptions and views, and is intended to provide more information. If there are copyright issues, please contact us, and we will address them promptly. This information is for healthcare professionals to understand the information and cannot replace professional medical guidance and should not be considered as medical advice. If this information is used for purposes other than understanding information, this platform and the authors do not bear related responsibilities.Contact email for cooperation:[email protected].
