[Research Background]
Aqueous zinc-ion batteries have good application prospects in large-scale energy storage due to their high safety and low cost. However, the corrosion of zinc metal in aqueous electrolytes, as well as the growth of zinc dendrites during deposition, leads to low coulombic efficiency and cycling stability of the zinc metal anode, severely hindering its large-scale application. Introducing cosolvents into trifluoromethanesulfonic acid zinc electrolytes is an effective method to address this issue, as this electrolyte has the potential to form a solid electrolyte interphase rich in ZnF2 and ZnS on the zinc metal surface. However, there is currently no simple method to directly reflect the interaction between solvent and solute, which hinders the rational design of the solvation structure of the electrolyte.
[Work Introduction]
Recently, Professor Weijie Li from Central South University and Professor Jiantie Xu from South China University of Technology proposed using logP as a standard for selecting cosolvents for trifluoromethanesulfonic acid zinc electrolytes and verified its universality by testing seven different types of solvents. In logP,P is the octanol-water partition coefficient, which describes the general parameter of hydrophilicity and hydrophobicity of chemical substances. Solvents with a logP value similar to that of the salt anion CF3SO3– can interact with CF3SO3–, Zn2+, and H2O, thereby reconstructing the solvation structure of the electrolyte. To further investigate the interactions between components in the modified electrolyte and their effects on the electrochemical performance of the battery, they conducted experiments and theoretical calculations using methyl acetate (MA) as an example. They found that MA molecules not only entered the solvation shell of CF3SO3–, but also coordinated with Zn2+ or H2O, forming a core-shell Zn2+ solvation structure containing MA and CF3SO3–. This special solvation structure reduces the activity of H2O and helps form a solid electrolyte interphase rich in ZnCO3-ZnF2 induced by anions on the zinc metal surface. Based on this, the hydrogen evolution reaction in the battery and the growth of zinc dendrites are effectively suppressed, significantly improving the cycling stability of zinc symmetrical batteries and full batteries. This article titled “Salt Anion Amphiphilicity-Activated Electrolyte Cosolvent Selection Strategy toward Durable Zn Metal Anode” was published in the internationally renowned journal ACS Nano. PhD student Liyang Liu is the first author, and Professors Weijie Li and Jiantie Xu are the corresponding authors.
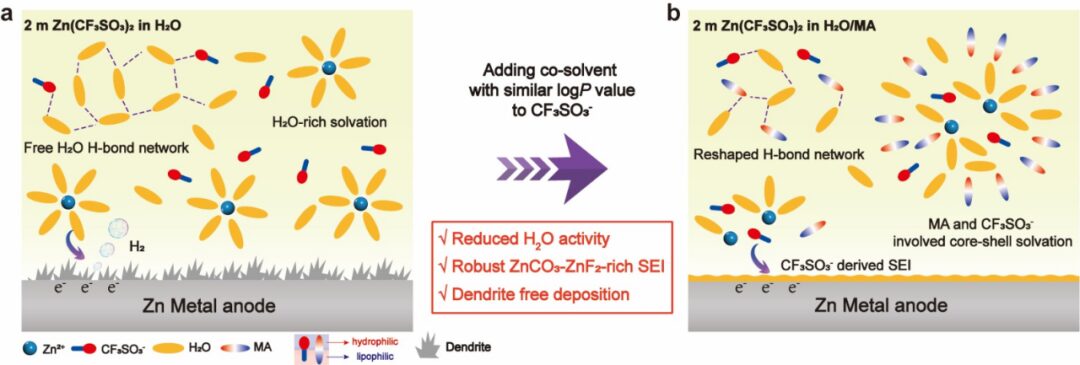
Figure 1. Schematic diagram showing the mechanism by which cosolvents with a logP value similar to that of CF3SO3– regulate the solvation structure of the electrolyte and the mechanism of SEI formation. (a) Trifluoromethanesulfonic acid zinc electrolyte and (b) solvation structure of trifluoromethanesulfonic acid zinc electrolyte using MA as a cosolvent and corresponding interfacial reactions.
[Content Description]
1. Selection of Cosolvent and Characterization of Solvation Structure
The authors chose carboxylic esters as the research object because they have a lower dielectric constant, which may increase the aggregation of cations and anions in the electrolyte when using carboxylic esters as cosolvents. Among carboxylic esters, the authors selected MA as the research object because: (i) MA has the closest logP value to CF3SO3– among all carboxylic esters, indicating that MA has similar amphiphilicity to CF3SO3–. According to the principle of similar solubility, MA and CF3SO3– are more likely to interact in an aqueous solution environment; (ii) MA has the smallest logP value among all carboxylic esters, indicating that MA has the highest hydrophilicity, thus MA is more likely to coordinate with H2O and Zn2+; (iii) MA has the smallest molecular size among all carboxylic esters. Based on the steric hindrance effect, MA is more likely to enter the solvation shell of Zn2+. NMR, infrared, Raman spectroscopy, and theoretical calculations indicate that MA can enter the solvation structure of CF3SO3–, thereby promoting the dissolution of MA in the electrolyte and the aggregation of CF3SO3– and Zn2+. At the same time, MA disrupts the hydrogen bonding network of H2O and coordinates with Zn2+, forming a core-shell Zn2+ solvation structure containing MA and CF3SO3–.
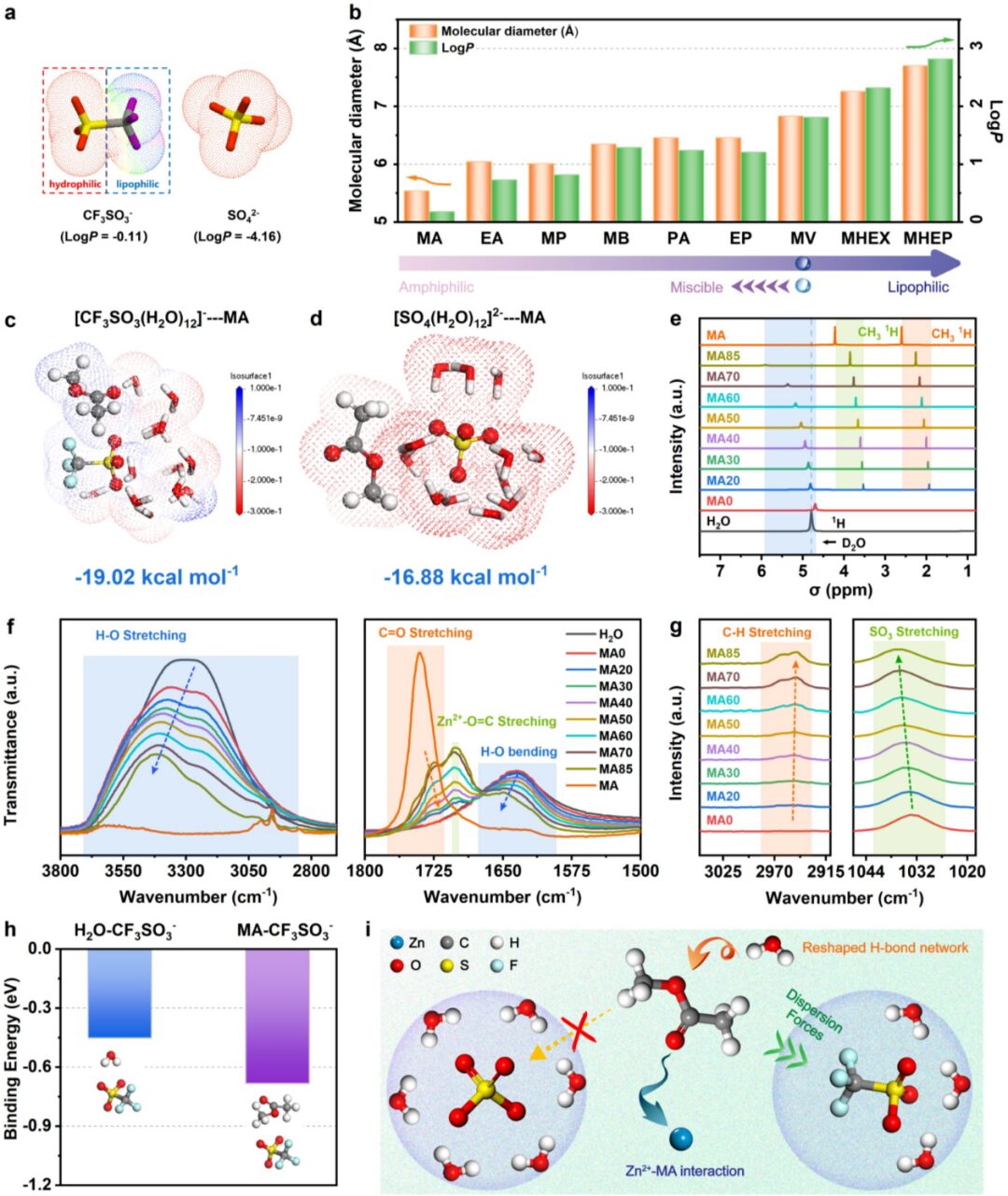
Figure 1. (a) Lipophilicity potential and logP values of CF3SO3– and SO42-. (b) LogP values and molecular diameters of different carboxylic esters. (c) [CF3SO3(H2O)12]–—MA and (d) [SO4(H2O)12]2-—MA structural optimization models and binding energies. MAx (x= 0, 20, 30, 40, 50, 60, 70 and 85), (e) NMR spectra of MA and H2O, (f) infrared spectra and (g) Raman spectra. (h) Binding energy of solvent-CF3SO3–. (i) Schematic diagram of the interaction mechanism between MA and the components of the electrolyte containing CF3SO3– and SO42-.
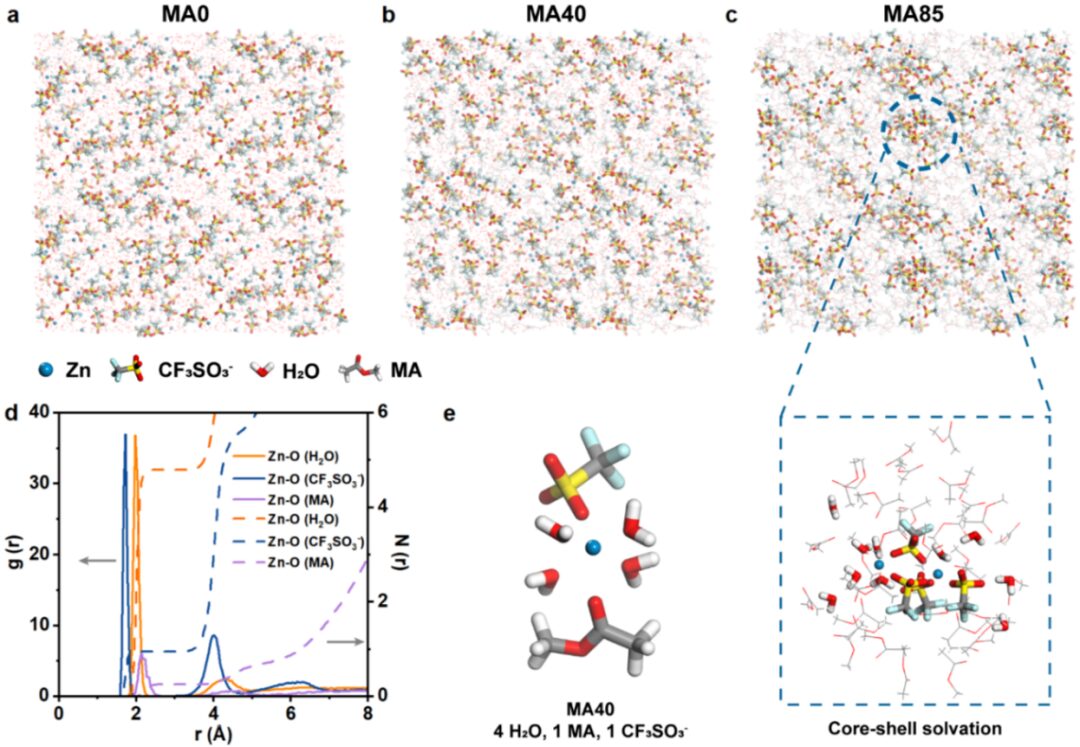
Figure 2. (a) Molecular dynamics simulation snapshots of MA0, (b) MA40, and (c) MA85 electrolytes. (d) Radial distribution function of MA40. (e) Solvation structure of Zn2+ in MA40.
2. The Effect of Cosolvent on the Reversibility of Zinc Metal Anode and the Properties of Electrolyte
The authors introduced seven solvents with a logP value similar to that of CF3SO3– into a 2 m trifluoromethanesulfonic acid zinc electrolyte and verified the universality of the cosolvent selection strategy by testing the coulombic efficiency of Zn||Ti batteries. Subsequently, the authors used MA40 as an example and verified the suppression effect of the modified electrolyte on the hydrogen evolution reaction and zinc dendrite growth by testing Tafel curves, CA curves, in situ observation of zinc plating, and measuring the amount of hydrogen generated during the zinc deposition process. The test results of the zinc symmetrical battery showed that the introduction of MA can significantly improve the cycling stability of the zinc metal anode. After 50 cycles, photos of the zinc metal anode, SEM images, XRD images, and XPS spectra further confirmed that MA40 can suppress the growth of zinc dendrites and side reactions and promote the formation of a solid electrolyte interphase rich in ZnCO3-ZnF2 on the surface of the zinc metal anode.
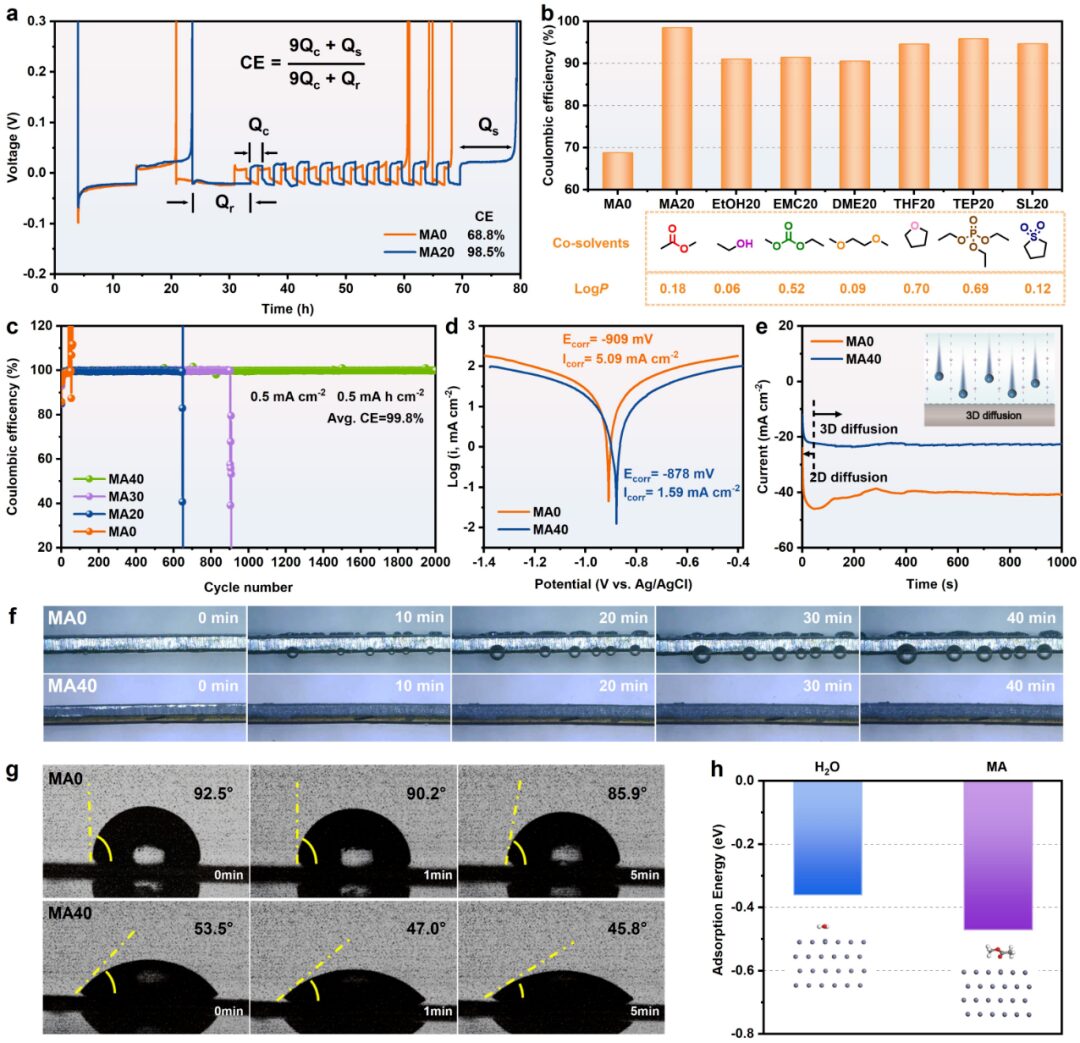
Figure 3. (a) Voltage-time curves of Zn//MAx//Ti (x= 0 and 20). (b) Coulombic efficiency graphs of Zn||Ti batteries using 2 m Zn(CF3SO3)2 electrolytes containing different cosolvents. (c) Coulombic efficiency curves of Zn//MAx//Cu (x= 0, 20, 30 and 40) batteries. (d) Tafel curves and (e) CA curves of zinc metal anodes measured in MAx (x= 0 and 40). (f) In situ optical microscopy of the zinc metal anode during plating in MAx (x= 0 and 40). (g) Contact angles of MA0 and MA40 on the zinc metal anode. (h) Adsorption energies of MA and H2O on the Zn(002) surface.
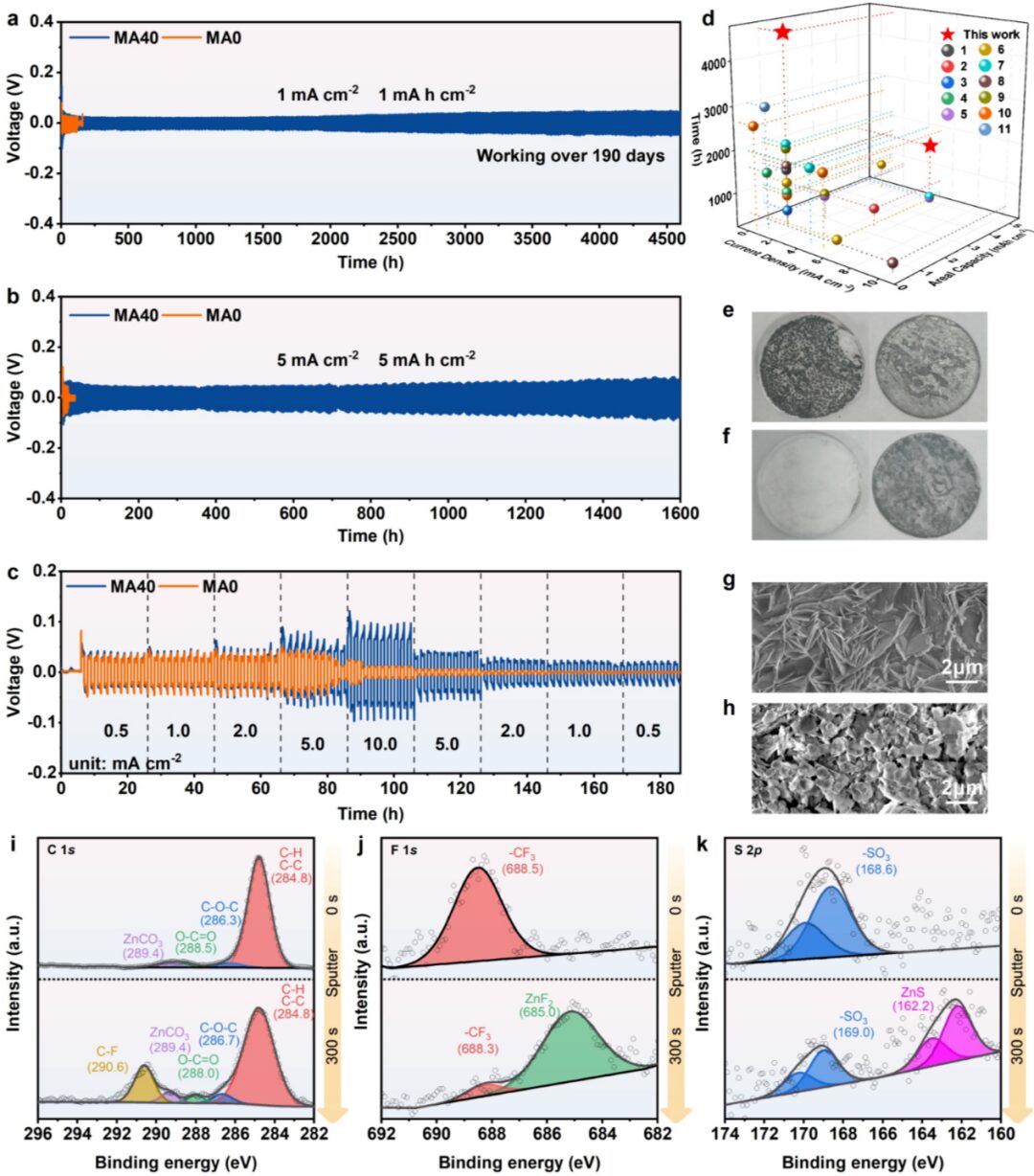
Figure 4. Voltage-time curves of Zn//MAx//Zn (x= 0 and 40) at (a) 1 mA cm-2, 1 mAh cm-2, (b) 5 mA cm-2, 5 mAh cm-2, and (c) under different current densities and areal capacities. (d) Comparison of electrochemical performance of Zn//MA40//Zn symmetrical battery with other zinc symmetrical batteries using modified electrolytes or modified zinc metal anodes. Under the conditions of 1 mA cm-2 and 1 mAh cm-2, (e) Photos of Zn//MA0//Zn and (f) Zn//MA40//Zn after 50 cycles, showing the separator (left) and the zinc metal anode (right). Under the conditions of 1 mA cm-2 and 1 mAh cm-2, (g) SEM images of Zn//MA0//Zn and (h) Zn//MA40//Zn after 50 cycles. After 50 cycles of Zn//MA40//Zn under 1 mA cm-2 and 1 mAh cm-2, high-resolution XPS spectra of the zinc metal anode surface and after Ar+ etching: (i) C 1s, (j) F 1s, and (k) S 2p.
3. Full Battery Performance Evaluation
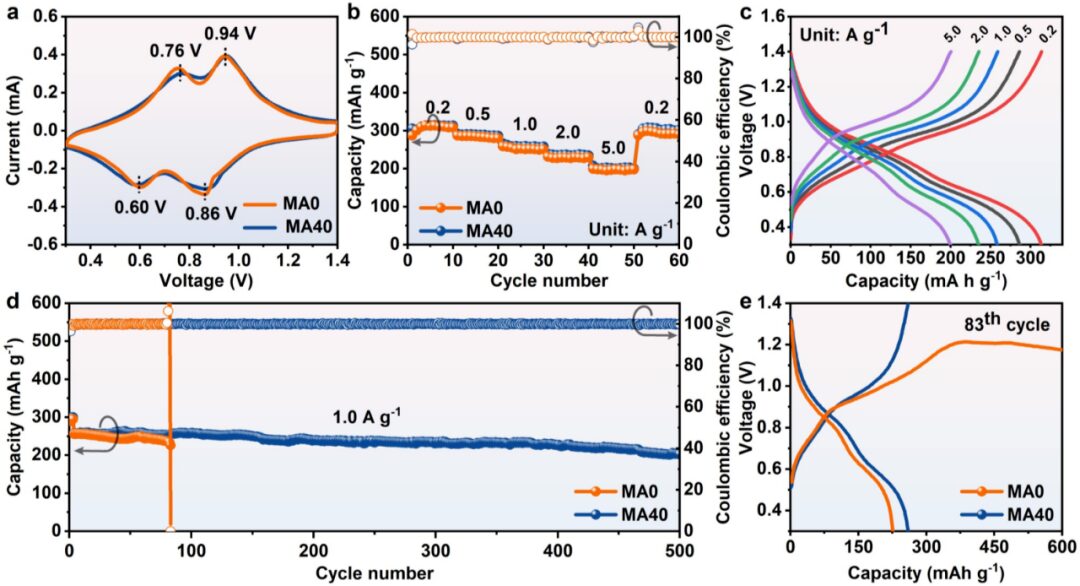
To further verify the effectiveness of MA40 in practical applications, the authors tested the rate performance and long cycle performance of Zn//MAx//NaV3O8·1.5H2O (NaV3O8·1.5H2O = NVO) (x = 0 and 40). The experimental results showed that MA40 not only slightly improved the rate performance of Zn||NVO batteries but also significantly improved their long cycle stability.
[Conclusion]
This paper proposes a convenient and effective standard for cosolvent selection (i.e., logP value) for rationally designing the solvation structure of trifluoromethanesulfonic acid zinc electrolytes. The authors quantified the hydrophilicity and hydrophobicity of the salt anion CF3SO3– and cosolvents using logP, introducing solvents with similar amphiphilicity to CF3SO3– (MA) into trifluoromethanesulfonic acid zinc electrolytes. Experimental and theoretical calculations indicate that MA molecules can enter the solvation structure of CF3SO3–, which not only aids in the dissolution of MA but also reduces the solubility of CF3SO3–. Additionally, MA molecules coordinate with Zn2+ or H2O, forming a core-shell Zn2+ solvation structure containing MA and CF3SO3–. This unique solvation structure not only helps to form a solid electrolyte interphase rich in ZnCO3-ZnF2 on the surface of the zinc metal anode but also helps to reconstruct the hydrogen bonding network of H2O, reducing the activity of free H2O. As a result, the hydrogen evolution reaction and the growth of zinc dendrites in the battery are suppressed. When using MA40 as the electrolyte in zinc symmetrical batteries, it exhibits ultra-high cycling stability under 1 mA cm-2 and 1 mAh cm-2, with a cycle life of up to 4600 hours. Furthermore, the Zn//MA40//NVO full battery can also stably operate for 500 cycles at 1 A g-1, with a capacity retention of 77.1%.
Liyang Liu, Haiying Lu, Chao Han, Xianfei Chen, Sucheng Liu, Jiakui Zhang, Xianghong Chen, Xinyi Wang, Rui Wang, Jiantie Xu, Hua Kun Liu, Shi Xue Dou, and Weijie Li. Salt Anion Amphiphilicity-Activated Electrolyte Cosolvent Selection Strategy toward Durable Zn Metal Anode, ACS Nano, 2023, DOI:10.1021/acsnano.3c08716
[Author Introduction]
Professor Weijie Li has focused on the theoretical and applied research of sodium-ion battery electrode materials (red phosphorus anodes and Prussian blue cathodes) with commercial application prospects during his PhD and postdoctoral periods. He was awarded the Discovery Early Career Research Award (DECRA) in Australia in 2018 and subsequently began independent research as a PI in aqueous zinc-ion batteries, with articles published in Advanced Energy Materials and Science Advances, among others. To date, he has published over 30 related SCI papers as the first author, co-first author, or corresponding author, including in Adv. Mater., Adv. Energy Mater., Sci. Adv., Nano Lett., ACS Nano, Chem. Mater., etc., with over 5500 citations and an h-index of 37 (Google Scholar).
Currently, Professor Li’s research group has completed the construction of the laboratory, equipped with instruments and equipment for material preparation and electrochemical testing. Moreover, the department has a series of high-end testing equipment related to electrochemistry, such as in situ XRD, DEMS, TOF-SIMS, SEM-FIB, etc., providing a complete setup for the preparation and testing of battery electrode materials. The research group mainly focuses on the development of high-performance sodium-ion batteries, zinc-ion battery electrode materials, and new energy storage devices. Interested students are welcome to join our research group, which is recruiting master’s, doctoral, and postdoctoral candidates.