Alkenes, as one of the core products in petrochemicals, are widely used in fields such as pharmaceuticals, materials, and chemicals. Alkenes play a key bridging role in medicinal chemistry, allowing for numerous transformations as needed.
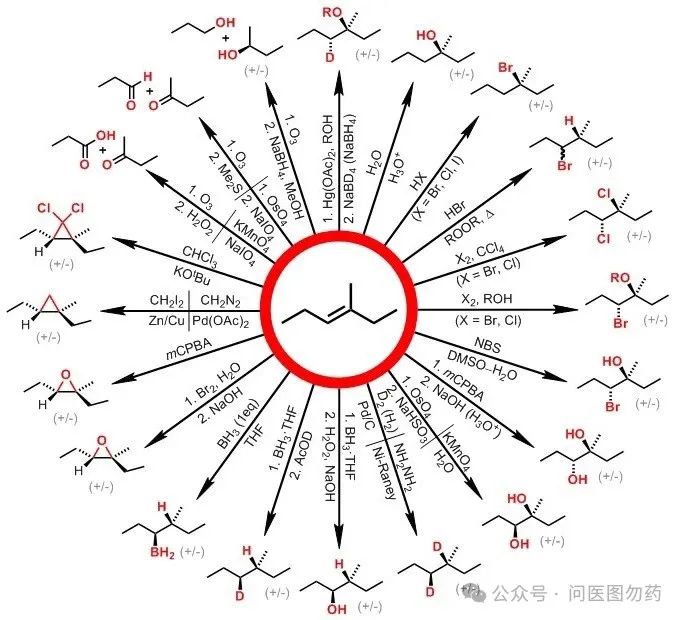
Among them, the well-known hydroboration reaction can achieve the transformation of alkenes to boronate esters.
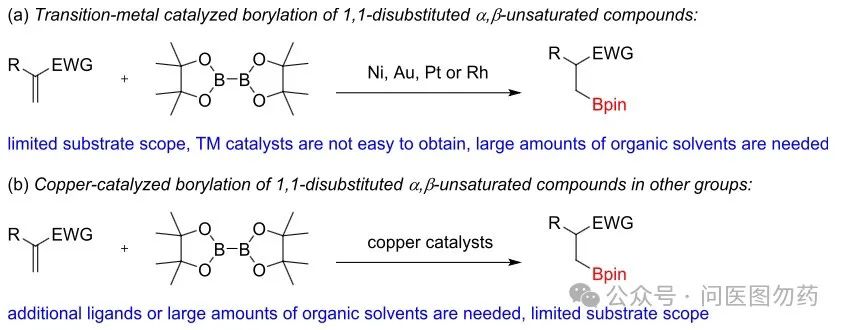
This issue will present several strategies for preparing bulky alkyl boronate esters from alkenes and their derivatives through transition metal activation. Readers can choose an appropriate catalytic system based on their needs and substrate characteristics.
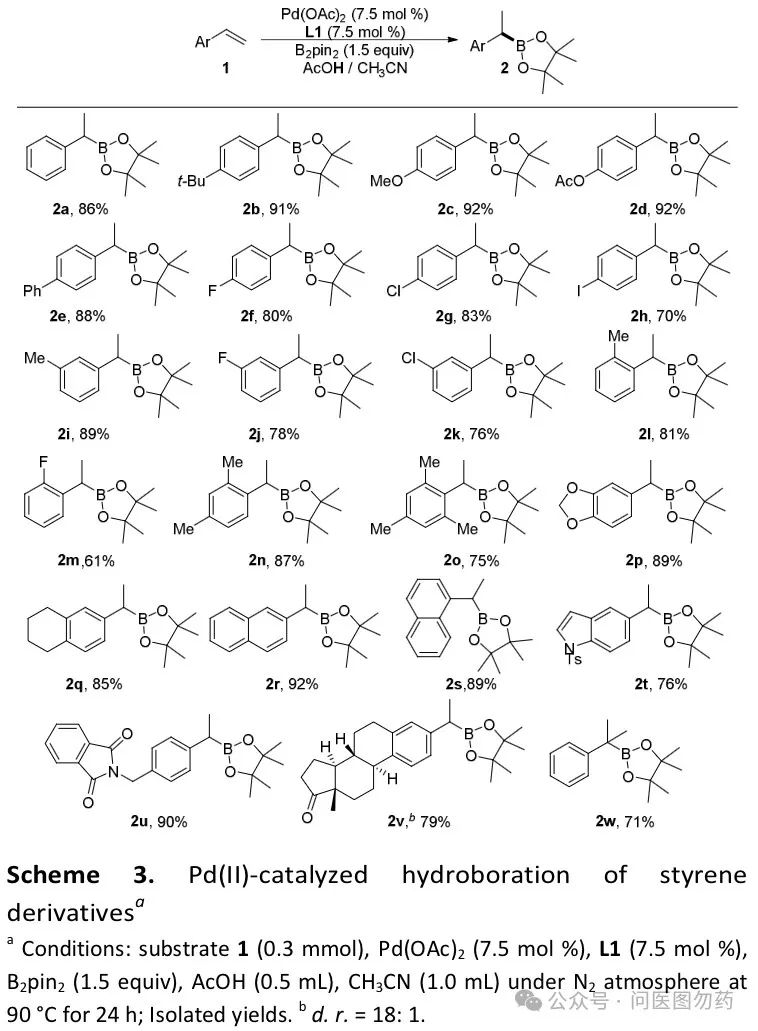
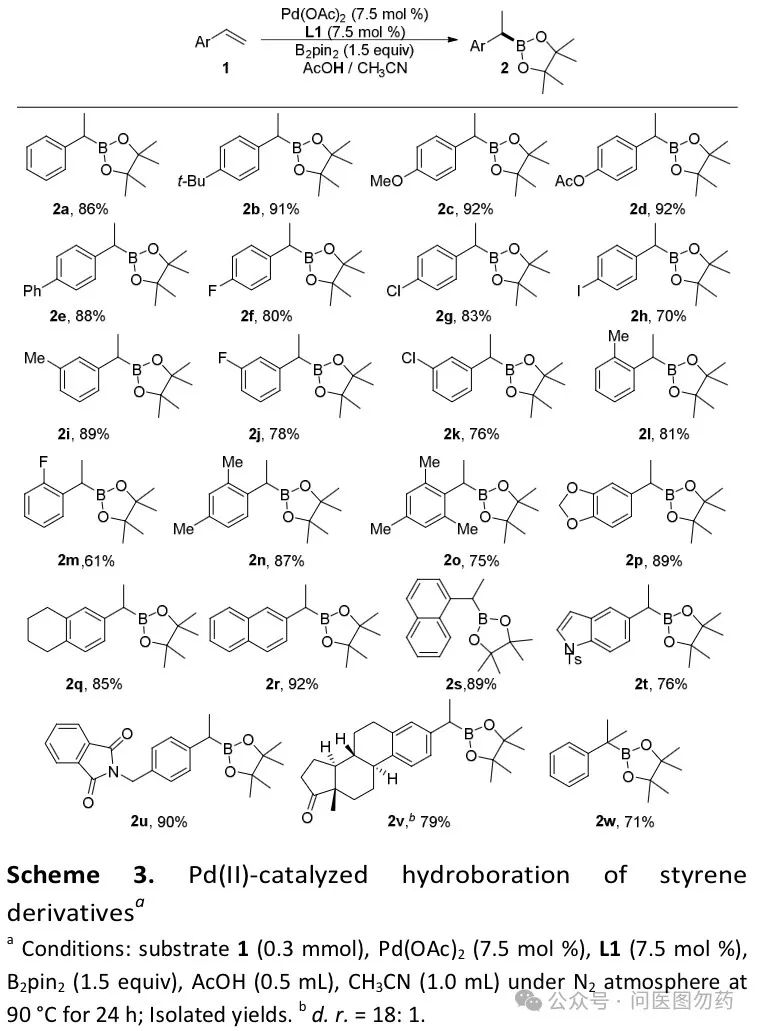
Example 1: Pd-catalyzed regioselective hydroboration of alkenes with B2Pin2 to construct bulky aryl boronate esters.
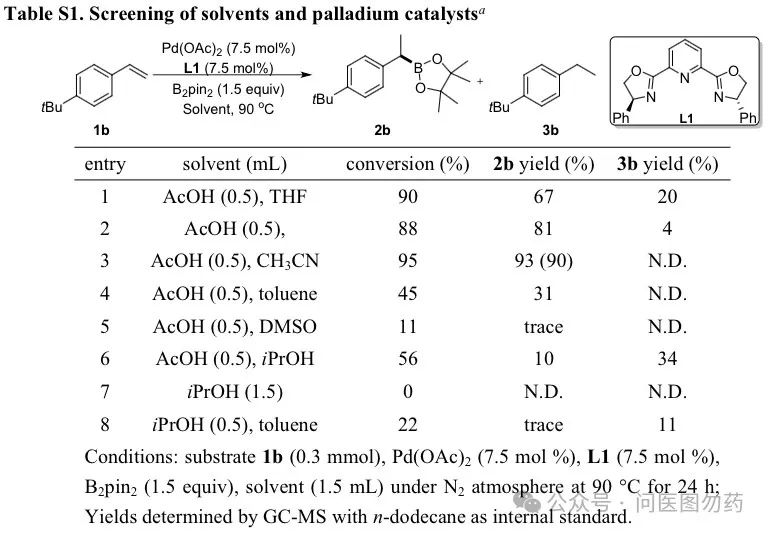
In 2018, Jiang Huanfeng et al. reported a method under mild conditions, using acetic acid as a solvent and hydrogen source, with palladium (II) catalyzing the hydroboration of aryl alkenes with (pinacol) diboron (B2pin2). This reaction features a broad substrate scope and high regioselectivity.
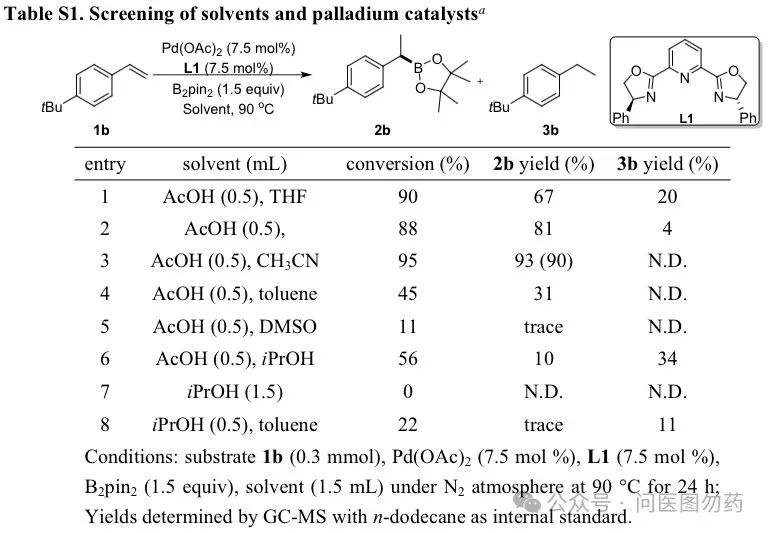
Optimization of Reaction Conditions
In a 20 mL sealed tube, Pd(OAc)2 (0.0225 mmol), L1 (0.0225 mmol), B2pin2 (0.45 mmol), solvent (1.5 mL), 1b (0.30 mmol) were successively added and vigorously stirred together at 90°C for 24 h under N2 atmosphere. After the reaction was finished, the mixture was cooled to room temperature. The reaction was neutralized with saturated NaHCO3 aq. and extracted with EtOAc (3 × 15 mL). The combined ethyl acetate layer was washed with brine (10 mL) and dried over anhydrous Na2SO4. The solvent was removed under vacuum. The crude product was purified by flash column chromatography (eluting with petroleum ether/ethyl acetate), and calculated the isolated yield.
The optimization results are as follows:
References
Palladium-catalyzed regioselective hydroboration of aryl alkenes with B2pin2. Chem. Commun. 2018, 54, 1770-1773. https://doi.org/10.1039/C7CC09432A
Example 2: Ni-catalyzed enantioselective 1,1-arylboration of terminal alkenes to prepare bulky alkyl boronate esters.
The enantioselective difunctionalization of alkenes is an effective strategy for assembling racemic or non-chiral building blocks to construct complex chiral molecules. Yin Guoyin et al. from Wuhan University reported a method for the enantioselective 1,1-arylboration of unactivated terminal alkenes using nickel catalysis. The study found that the high regio- and enantioselectivity of the reaction is due to the directional selection of the nickel catalyst, rather than the introduction of directing groups. Furthermore, allylbenzene also maintained excellent regioselectivity.
A 100 mL oven-dried round-bottom flask equipped with a magnetic stir bar was sealed with a septum and cooled under a stream of nitrogen. The septum was partly removed and the diamine ligand L7 ((IR,2R)-N,N-Dimethyl-1,2-cyclohexanediamine)(426.7 mg, 3.0 mmol, 1.0 equiv) and anhydrous NiCl2•DME (659.2 mg, 3.0 mmol, 1.0 equiv) were added, then anhydrous dioxane (30 mL) was added under nitrogen. The reaction mixture was stirred under nitrogen at rt overnight. After the reaction, the insoluble particles were removed using filtration through filter paper and the filtrate was concentrated under reduced pressure to get the precatalyst rac-[Ni]-1/[Ni]-1*.
Gram-Scale Reactions
Under N2 atmosphere, an oven-dried 120 mL reaction tube which equipped with a magnetic stir bar and sealed with a rubber stopper sequentially was added rac-[Ni]-1 (91 mg, 0.25 mmol, 5 mol%), LiOMe (290 mg, 7.5 mmol, 1.5 equiv), bis(pinacolato)diboron (1910 mg, 7.5 mmol, 1.5 equiv). Then anhydrous dioxane (20 mL), alkenes (5 mmol, 1.0 equiv), arylbromide (7.5 mmol, 1.5 equiv) were added and the mixture was stirred. After 30 h of stirring at 50°C, an aliquot of 20 uL was removed and analyzed by GC. The reaction vial was diluted with 10 mL of ethyl acetate and the resulting solution was filtered through Celite. The crude material was concentrated in vacuo and separated on a silica gel column affording the cross-coupling product. The synthesis of chiral products at 30°C, the ee values were determined by HPLC on a chiral stationary phase.
References
Catalyst-controlled enantioselective 1,1-arylboration of unactivated olefins. Nature Catalysis, 2020, 3, 951-958. https://doi.org/10.1038/s41929-020-00523-8
Example 3: Regioselective monoboration of spirocyclobutenes to prepare bulky alkyl boronate esters.
In 2021, Mariola Tortosa et al. reported a strategy for the regioselective monoboration of spirocyclobutenes catalyzed by a CuCl/Xantphos system to prepare bulky alkyl boronate esters. This strategy features a broad substrate applicability and excellent regioselectivity of the products.
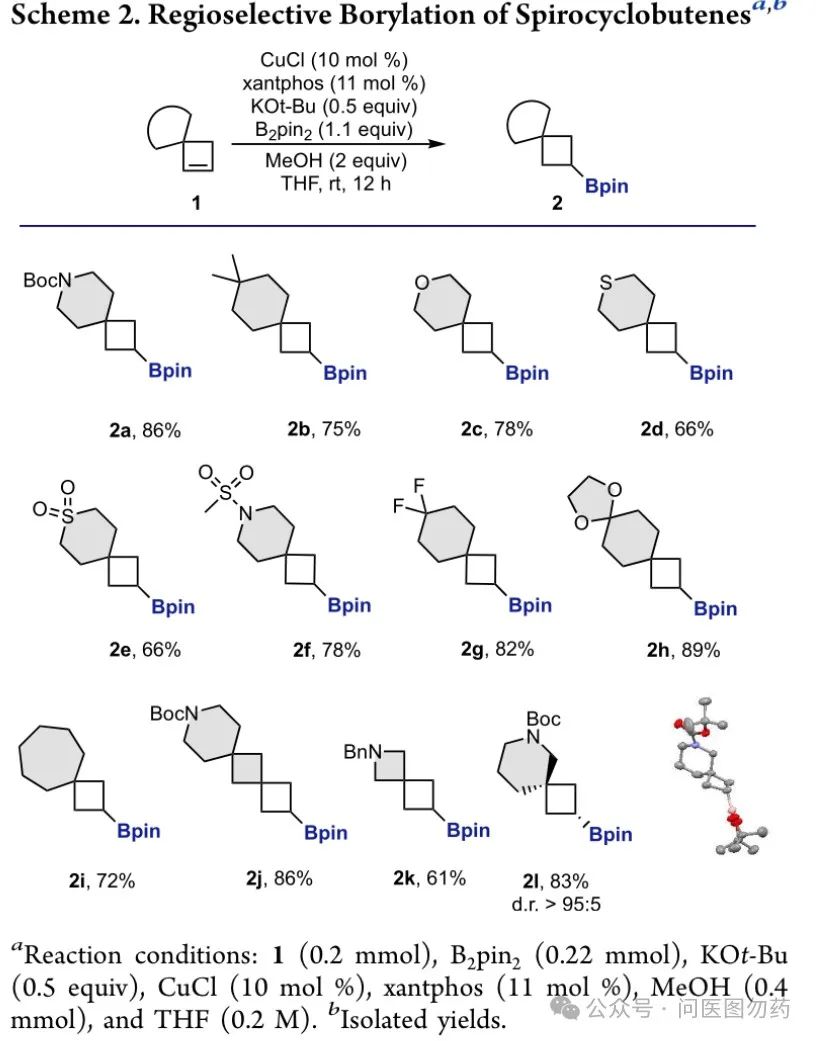
An oven-dried vial was charged with CuCl (10 mol%), B2pin2 (1.1 equiv), KOt-Bu (0.5 equiv) and xantphos (11 mol%) in the glove box. Anhydrous THF (0.5 mL/0.2 mmol of 1) was added and the mixture was stirred for 15 min. Then, the corresponding cyclobutene 1 (1.0 equiv) in THF (1 mL/0.2 mmol of 1) was added dropwise followed by methanol (2 equiv). Finally, the reaction mixture was stirred overnight at room temperature. Once the reaction was finished, the resulting solution was filtered through a pad of Celite® (eluted with EtOAc) and concentrated under reduced pressure. The crude product was purified by flash column chromatography on silica gel or Florisil® to afford cyclobutylboronate 2.
References
Regioselective Monoborylation of Spirocyclobutenes. Org. Lett. 2021, 23, 19, 7434–7438. https://doi.org/10.1021/acs.orglett.1c02645
Example 4: Cu2(OH)2CO3 catalyzed hydroboration of terminal alkenes to prepare bulky alkyl boronate esters.
Zhu Lei et al. reported a strategy for the enantioselective 1,1-hydroboration of unactivated terminal alkenes catalyzed by Cu2(OH)2CO3 to construct bulky alkyl boronate esters. The substrates can be extended to 1,1-disubstituted α,β-unsaturated ketones, esters, and amides, all showing excellent reactivity.
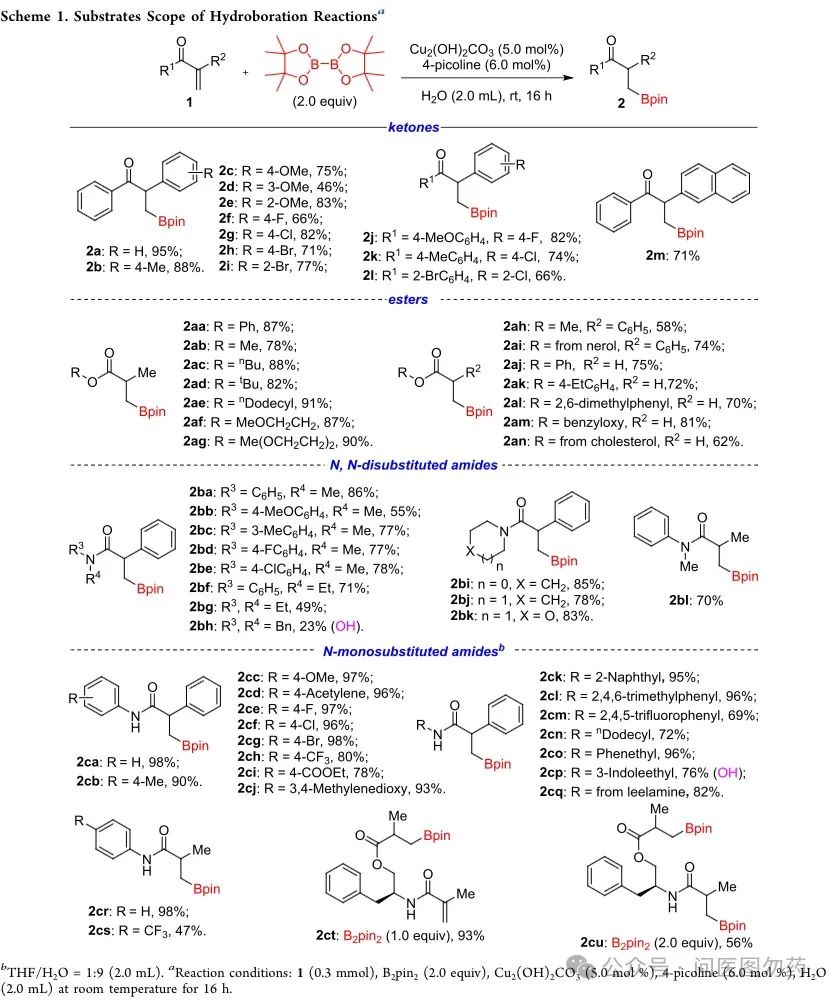
General Procedure for Cu2(OH)2CO3 Catalyzed Hydroboration Reactions of 1,1-Disubstituted α,β-Unsaturated Compounds
To a 3.0 mL vial equipped with a magnetic stirring bar were added 1,2-diphenylprop-2-en-1-one 1a (0.3 mmol), B2pin2 (0.6 mmol), Cu2(OH)2CO3 (0.015 mmol), 4-picoline (0.018 mmol) and 2.0 mL of H2O, the whole reaction was then stirred at room temperature for 16 h. After completion of the reaction, filtered through a celite pad, washed with ethyl acetate (EtOAc, 5 mL), separated, and the water phase was extracted with EtOAc (5 mL×3), combined with organic phase, then washed with brine, separated and dried over anhydrous Na2SO4, the organic solvent was removed under reduced pressure to obtain the crude product, the final product 2a (95.7 mg, 0.29 mmol) was purified as a white solid by silica gel chromatography (PE: EA = 30:1) in 95% yield. 1H NMR (400 MHz, CDCl3) δ 7.97-7.88 (m, 2H), 7.44 (t, J = 7.4 Hz, 1H), 7.34 (t, J = 7.6 Hz, 2H), 7.30-7.20 (m, 4H), 7.20-7.11 (m, 1H), 4.79 (dd, J = 9.3, 6.7 Hz, 1H), 1.57 (dd, J = 15.9, 9.3 Hz, 1H), 1.34 (dd, J = 15.9, 6.7 Hz, 1H), 1.19 (s, 6H), 1.12 (s, 6H); 13C{1H} NMR (100 MHz, CDCl3) δ 200.7, 142.0, 136.6, 132.5, 128.92, 128.89, 128.3, 128.0, 126.7, 83.3, 50.2, 24.8, 24.6.
References
Cu(II)-Catalyzed Hydroboration Reactions of 1,1-Disubstituted α,β-Unsaturated Ketones, Esters, and Amides in Pure Water. J. Org. Chem. 2024, 89, 12, 8334–8341. https://doi.org/10.1021/acs.joc.3c02942
Example 5: Mg-mediated synthesis of bulky alkyl boronate esters from α,α-disubstituted allyl bromides.
In 2014, James P. Morken et al. reported strategies using Pd and Mg to synthesize bulky alkyl boronate esters from allyl bromides, comparing the different functions and regioselectivity of the two reagents.
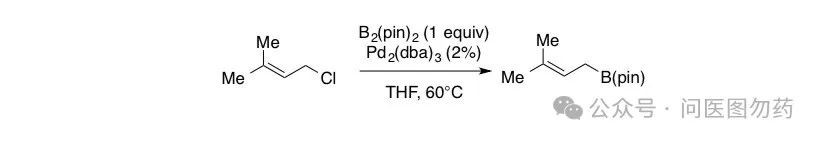
An oven-dried scintillation vial equipped with a magnetic stir bar was charged with Pd2(dba)3 (54.9 mg, 0.06 mmol), bis(pinacolato)diboron (762.6 mg, 3.00 mmol), and tetrahydrofuran (1.5 mL) in a dry-box under argon atmosphere. The vial was capped and stirred for two minutes, then 1-chloro-3-methylbut-2-ene (313.7 mg, 3.00 mmol) was added. The vial was capped with a teflon cone-lined cap, sealed with electrical tape, removed from the dry-box, and heated to 60°C and allowed to stir for 12 h. The reaction was then concentrated in vacuo and the crude reaction mixture was purified rapidly on oven-dried silica gel (fast gradient of 50:1-20:1 pentane:diethyl ether) to afford a clear, colorless oil (364 mg, 62% yield). Rf= 0.53 (20:1 pentane :diethyl ether, stain in KMnO4). Spectral data is in accordance with the literature.
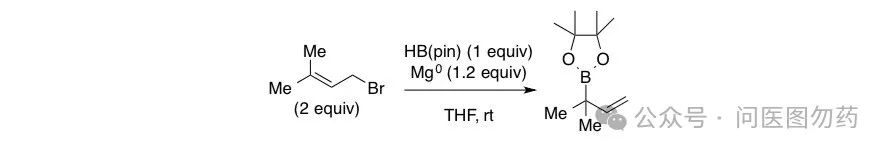
Representative Procedure
An oven-dried round-bottomed flask equipped with a magnetic stir bar was charged with magnesium turnings (292 mg, 12.0 mmol), pinacol borane (HBpin) (1.28 g, 10.0 mmol), and tetrahydrofuran (15 mL) in a dry-box under argon atmosphere. One equivalent of 1-bromo-3-methylbut-2-ene (1.49 g, 10.0 mmol) was added dropwise over 5 minutes with stirring, and the solution was capped and stirred for an additional 30 minutes. A second equivalent of 1-bromo-3-methylbut-2-ene (1.49 g, 10.0 mmol) was added dropwise, and the solution was sealed with a septa and electrical tape, removed from the dry-box, and stirred under nitrogen for an additional 2 hours at room temperature. The solution was then cooled to 0°C, diluted with hexanes (50 mL) and quenched with dropwise addition of aqueous 0.1 M HCl (90 mL). After stirring for 10 minutes, the solution was transferred to a separatory funnel and extracted with hexanes (3 x75 mL). The combined organics were dried over magnesium sulfate, filtered, and concentrated in vacuo. The crude reaction mixture was purified by kugelrohr distillation (hi-vacuum, 80°C) to afford a clear, colorless oil (1.42 g, 72% yield)(Compound 14). Spectral data is in accordance with the literature.
References
Congested C–C Bonds by Pd-Catalyzed Enantioselective Allyl–Allyl Cross-Coupling, a Mechanism-Guided Solution. J. Am. Chem. Soc. 2014, 136, 19, 7092–7100. https://doi.org/10.1021/ja502280w