 【Research Highlights】The lithium-oxygen battery, despite its high theoretical energy density (3500Wh/kg), still faces significant challenges for commercial application. One major challenge in charging lithium-oxygen batteries is the low charging current, high overpotential, numerous side reactions, incomplete charging decomposition, and low coulombic efficiency. The discharge product lithium peroxide (Li2O2) is an insulating solid, which complicates the solid-solid contact issue between the catalyst and lithium peroxide during charging. Over the past decade, researchers worldwide have attempted various strategies to address the high charging overpotential (high polarization) of lithium-oxygen batteries by developing numerous charging catalysts aimed at reducing the overpotential, primarily to accelerate the decomposition kinetics of lithium peroxide. However, when we discuss the charging overpotential of lithium-oxygen batteries, where does it actually originate? Is the decomposition kinetics of lithium peroxide the bottleneck? If the kinetics are slow, what is their contribution to the overall overpotential? Besides the decomposition kinetics of lithium peroxide, many other factors can lead to charging overpotential, such as contact resistance (poor interfacial contact), mass transfer limitations within the electrode pores, slow Li+ transport in the SEI layer, overpotential caused by passivation of the electrode surface by side products like lithium carbonate, and slow decomposition kinetics of lithium carbonate, among others. Is the decomposition of lithium peroxide truly the kinetic bottleneck? If not, why design catalysts specifically for the lithium peroxide decomposition reaction? Therefore, clarifying the sources of charging overpotential is crucial for the design of electrocatalysts. However, many current in-situ methods focus solely on the dynamic changes in composition and structure during charging, making in-situ monitoring methods that can simultaneously consider changes in composition, structure, and electrochemical performance more comprehensive and reliable. Electrochemical impedance spectroscopy (EIS) can perform in-situ monitoring without harming the battery itself, and the impedance of each process directly corresponds to the charging overpotential. By using the distribution of relaxation times (DRT) and distribution of capacitance times (DCT) methods, the high-frequency and low-frequency parts of the impedance spectrum can be analyzed separately, distinguishing multiple interfacial processes based on characteristic time constants and tracking their changes during the charging process, revealing the main sources of charging overpotential. 【Main Content】Recently, Professor Chen Yuhui from Nanjing University of Technology and Professor Francesco Ciucci from the Hong Kong University of Science and Technology (currently at the University of Bayreuth, Germany) monitored the charging process of lithium-oxygen batteries using in-situ electrochemical impedance spectroscopy (in-situ EIS) combined with in-situ electrochemical mass spectrometry (in-situ DEMS). They further analyzed the impedance results using methods such as distribution of relaxation times (DRT) and distribution of capacitance times (DCT), revealing the charging mechanism of lithium-oxygen batteries and clarifying the sources of charging overpotential, providing guidance for the design and optimization of catalysts. During the charging process of the composite electrode, there exists a competitive relationship between the decomposition of lithium peroxide and lithium carbonate. It was found that at the beginning and end of charging, the charging overpotential mainly originates from the charge transfer of lithium peroxide decomposition; while in the middle of charging, the overpotential mainly comes from the decomposition of lithium carbonate, which overcomes the passivation of the catalyst surface by lithium carbonate. The decomposition of lithium carbonate in the middle of charging is critical because its decomposition generates reactive species that exacerbate electrolyte decomposition, and the resulting byproducts can clog the electrode pores, hindering the diffusion of products on the composite electrode. The related article titled “Charging Processes in Lithium-Oxygen Batteries Unraveled through the Lens of the Distribution of Relaxation Times” was published in Chem. 【Illustrative Analysis】1. Changes in electrochemical impedance and gas evolution during chargingThe authors assembled a lithium-oxygen battery with a composite electrode of Super P-PTFE-Co3O4 and monitored the impedance changes and gas evolution at different stages of charging. To simulate the actual daily charge and discharge behavior, commercially available nano Co3O4 was directly chosen as the catalyst without optimizing the electrolyte and charge-discharge conditions, thus avoiding many side reactions. A significant amount of CO2 gas was observed during charging, indicating the generation and decomposition of many lithium carbonate or organic carbonate byproducts. The results showed that the amount of O2 generated increased→decreased→increased→decreased, while the amount of CO2 first increased and then decreased, and the impedance semicircle also showed a trend of first increasing and then decreasing.
【Research Highlights】The lithium-oxygen battery, despite its high theoretical energy density (3500Wh/kg), still faces significant challenges for commercial application. One major challenge in charging lithium-oxygen batteries is the low charging current, high overpotential, numerous side reactions, incomplete charging decomposition, and low coulombic efficiency. The discharge product lithium peroxide (Li2O2) is an insulating solid, which complicates the solid-solid contact issue between the catalyst and lithium peroxide during charging. Over the past decade, researchers worldwide have attempted various strategies to address the high charging overpotential (high polarization) of lithium-oxygen batteries by developing numerous charging catalysts aimed at reducing the overpotential, primarily to accelerate the decomposition kinetics of lithium peroxide. However, when we discuss the charging overpotential of lithium-oxygen batteries, where does it actually originate? Is the decomposition kinetics of lithium peroxide the bottleneck? If the kinetics are slow, what is their contribution to the overall overpotential? Besides the decomposition kinetics of lithium peroxide, many other factors can lead to charging overpotential, such as contact resistance (poor interfacial contact), mass transfer limitations within the electrode pores, slow Li+ transport in the SEI layer, overpotential caused by passivation of the electrode surface by side products like lithium carbonate, and slow decomposition kinetics of lithium carbonate, among others. Is the decomposition of lithium peroxide truly the kinetic bottleneck? If not, why design catalysts specifically for the lithium peroxide decomposition reaction? Therefore, clarifying the sources of charging overpotential is crucial for the design of electrocatalysts. However, many current in-situ methods focus solely on the dynamic changes in composition and structure during charging, making in-situ monitoring methods that can simultaneously consider changes in composition, structure, and electrochemical performance more comprehensive and reliable. Electrochemical impedance spectroscopy (EIS) can perform in-situ monitoring without harming the battery itself, and the impedance of each process directly corresponds to the charging overpotential. By using the distribution of relaxation times (DRT) and distribution of capacitance times (DCT) methods, the high-frequency and low-frequency parts of the impedance spectrum can be analyzed separately, distinguishing multiple interfacial processes based on characteristic time constants and tracking their changes during the charging process, revealing the main sources of charging overpotential. 【Main Content】Recently, Professor Chen Yuhui from Nanjing University of Technology and Professor Francesco Ciucci from the Hong Kong University of Science and Technology (currently at the University of Bayreuth, Germany) monitored the charging process of lithium-oxygen batteries using in-situ electrochemical impedance spectroscopy (in-situ EIS) combined with in-situ electrochemical mass spectrometry (in-situ DEMS). They further analyzed the impedance results using methods such as distribution of relaxation times (DRT) and distribution of capacitance times (DCT), revealing the charging mechanism of lithium-oxygen batteries and clarifying the sources of charging overpotential, providing guidance for the design and optimization of catalysts. During the charging process of the composite electrode, there exists a competitive relationship between the decomposition of lithium peroxide and lithium carbonate. It was found that at the beginning and end of charging, the charging overpotential mainly originates from the charge transfer of lithium peroxide decomposition; while in the middle of charging, the overpotential mainly comes from the decomposition of lithium carbonate, which overcomes the passivation of the catalyst surface by lithium carbonate. The decomposition of lithium carbonate in the middle of charging is critical because its decomposition generates reactive species that exacerbate electrolyte decomposition, and the resulting byproducts can clog the electrode pores, hindering the diffusion of products on the composite electrode. The related article titled “Charging Processes in Lithium-Oxygen Batteries Unraveled through the Lens of the Distribution of Relaxation Times” was published in Chem. 【Illustrative Analysis】1. Changes in electrochemical impedance and gas evolution during chargingThe authors assembled a lithium-oxygen battery with a composite electrode of Super P-PTFE-Co3O4 and monitored the impedance changes and gas evolution at different stages of charging. To simulate the actual daily charge and discharge behavior, commercially available nano Co3O4 was directly chosen as the catalyst without optimizing the electrolyte and charge-discharge conditions, thus avoiding many side reactions. A significant amount of CO2 gas was observed during charging, indicating the generation and decomposition of many lithium carbonate or organic carbonate byproducts. The results showed that the amount of O2 generated increased→decreased→increased→decreased, while the amount of CO2 first increased and then decreased, and the impedance semicircle also showed a trend of first increasing and then decreasing.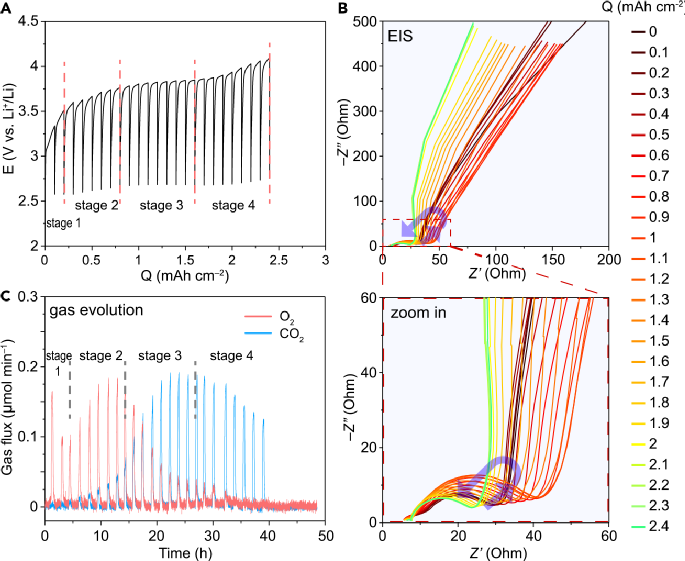 Figure 1 Charging curve of the lithium-oxygen battery with Super P-PTFE-Co3O4 as the composite electrode. (a) Charging curve with 1M LiTFSI TEGDME as the electrolyte, (b) Nyquist plot of impedance changes at different stages of charging, (c) gas evolution of O2 and CO2 at different stages of charging Before charging at OCV, the DRT corresponding to the EIS of the lithium-oxygen battery had four peaks in the time constant range of 10-6~10-1s, corresponding to four main electrode processes or reactions, denoted as τ1-τ4. The overall trend shows that the peaks τ1 and τ2 gradually decrease, while τ3 and τ4 peaks first increase and then decrease, presenting an “inverted” trend, which is the main reason for the overall increase and then decrease of impedance shown in Figure 1. Therefore, it is necessary to clarify the attribution of these peaks.
Figure 1 Charging curve of the lithium-oxygen battery with Super P-PTFE-Co3O4 as the composite electrode. (a) Charging curve with 1M LiTFSI TEGDME as the electrolyte, (b) Nyquist plot of impedance changes at different stages of charging, (c) gas evolution of O2 and CO2 at different stages of charging Before charging at OCV, the DRT corresponding to the EIS of the lithium-oxygen battery had four peaks in the time constant range of 10-6~10-1s, corresponding to four main electrode processes or reactions, denoted as τ1-τ4. The overall trend shows that the peaks τ1 and τ2 gradually decrease, while τ3 and τ4 peaks first increase and then decrease, presenting an “inverted” trend, which is the main reason for the overall increase and then decrease of impedance shown in Figure 1. Therefore, it is necessary to clarify the attribution of these peaks.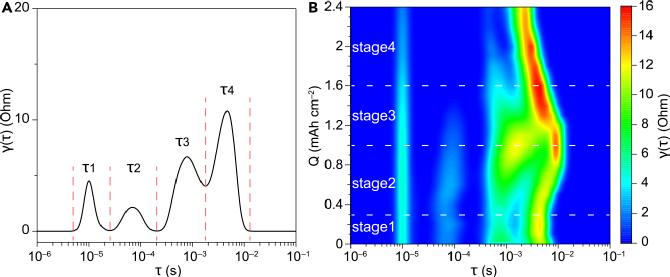 Figure 2 DRT plot corresponding to EIS during charging. (a) DRT plot of the composite electrode before charging in the lithium-oxygen battery, (b) DRT results at different charging stages 2. Confirming the attribution and changes of peaks in the DRT plotBy designing a series of control experiments, we confirmed the peaks in the DRT plot. τ1 corresponds to contact impedance, τ2 corresponds to the impedance of Li+ passing through the SEI layer, τ3 corresponds to the charge transfer impedance of the decomposition reaction of carbonate products, and τ4 corresponds to the charge transfer impedance of the decomposition reaction of Li2O2. Additionally, the low-frequency region is more suitable for DCT processing, where τ5 in the DCT plot corresponds to the admittance of diffusion in the porous electrode. By observing the changes of each peak during the charging process, it can be found that at the beginning of charging, amorphous Li2O2 first decomposes to release O2, and then crystalline Li2O2 begins to decompose. As the charging voltage increases, Li2CO3 begins to decompose, and the amount of CO2 evolved increases. Since the decomposition of Li2CO3 releases singlet oxygen1O2, which then attacks the electrolyte, producing byproducts that clog the porous electrode. Therefore, during the middle of charging, the passivation of Li2CO3 is the main reason for the increase in charging overpotential. At the end of charging, the charge transfer difficulty of Li2O2 decomposition leads to an increase in charging overpotential.
Figure 2 DRT plot corresponding to EIS during charging. (a) DRT plot of the composite electrode before charging in the lithium-oxygen battery, (b) DRT results at different charging stages 2. Confirming the attribution and changes of peaks in the DRT plotBy designing a series of control experiments, we confirmed the peaks in the DRT plot. τ1 corresponds to contact impedance, τ2 corresponds to the impedance of Li+ passing through the SEI layer, τ3 corresponds to the charge transfer impedance of the decomposition reaction of carbonate products, and τ4 corresponds to the charge transfer impedance of the decomposition reaction of Li2O2. Additionally, the low-frequency region is more suitable for DCT processing, where τ5 in the DCT plot corresponds to the admittance of diffusion in the porous electrode. By observing the changes of each peak during the charging process, it can be found that at the beginning of charging, amorphous Li2O2 first decomposes to release O2, and then crystalline Li2O2 begins to decompose. As the charging voltage increases, Li2CO3 begins to decompose, and the amount of CO2 evolved increases. Since the decomposition of Li2CO3 releases singlet oxygen1O2, which then attacks the electrolyte, producing byproducts that clog the porous electrode. Therefore, during the middle of charging, the passivation of Li2CO3 is the main reason for the increase in charging overpotential. At the end of charging, the charge transfer difficulty of Li2O2 decomposition leads to an increase in charging overpotential.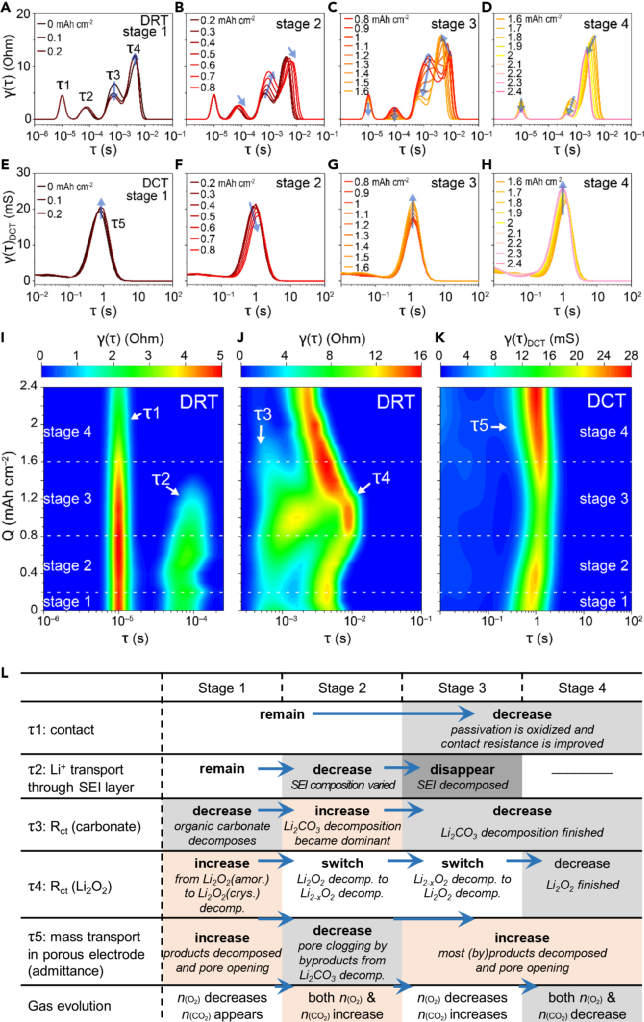 Figure 3 DRT and DCT plots corresponding to impedance during charging. (a-d) DRT plots for charging stages 1-4, (e-h) DCT plots for charging stages 1-4, (i-k) contour plots corresponding to DRT and DCT for charging stages 1-4, (I) Summary of peak changes and mechanisms of DRT and DCT at different charging stages Since DRT is mainly used in fuel cell systems, where reactions are singular and side reactions are few, even when applied to battery systems, it is mostly used to assess the growth of the SEI layer and battery degradation, with DRT peaks showing a relatively simple trend of change (increase/decrease), corresponding to the increase and decrease of impedance R values. Given that the electrode area remains unchanged, it can generally be considered related to the thickening or property changes of the SEI layer. However, in the lithium-oxygen system, the electrode reactions and interfacial processes are more complex, with multiple competing processes, and the active sites of the catalyst are limited. The increase and decrease of DRT peaks not only correspond to changes in reaction Rct but may also be due to one reaction gradually replacing another, becoming the main reaction (or process) on that catalyst.As shown in Figure 4, on a given area of the electrode (corresponding to a given total amount of active sites), when reaction 1 (blue block) transitions to reaction 2 (orange block), various changes in DRT peaks will occur depending on R1/R2 and C1/C2, not necessarily following the trends of ↑↓ and ↗↙. For example, the direct decomposition of Li2O2 or delithiation tends to be visually represented in the DRT plot as the main reaction rather than the one with slow kinetics (even if a reaction is slow, if its proportion is small, it will not be reflected in the EIS). Applied to the charging process of lithium-oxygen batteries, although both Super P carbon black and the catalyst surface can decompose Li2O2, these two processes are competitive; the decomposition reaction on the catalyst surface is much faster than that on carbon black, thus the reaction on the carbon black surface contributes little to the EIS spectrum.As shown in Figure 3, in the early stage of the reaction (stage 1), the overall impedance is small, and the overpotential is not large. Although the decomposition kinetics of Li2O2 slightly increase, the main contribution still comes from the passivation of carbonates. In the early stage of the reaction (stage 2), as the decomposition of organic carbonates between Li2O2 and the catalyst increases, the decomposition of Li2O2 occupies more catalyst sites, becoming the main reaction and the primary source of overpotential. At this time, the properties of the SEI layer also undergo some changes, and the decomposition of lithium carbonate leads to side reactions (such as singlet oxygen, etc.), causing some side reactions in the electrode pores, affecting the diffusion process. In the later stage of the reaction (stage 3), as the decomposition of Li2O2 consumes, its contribution to impedance and overpotential decreases, and the main reaction transitions from Li2O2 decomposition to lithium carbonate decomposition, with the potential gradually increasing, and the SEI layer also begins to decompose, enhancing the overall conductivity of the electrode. In the final stage of the reaction (stage 4), the Li2O2 that can be reached on the catalyst surface and the easily decomposable SEI have all decomposed, leaving behind substances that are difficult to decompose, such as lithium carbonate, which at this point becomes the main source of overpotential due to its slow decomposition kinetics.
Figure 3 DRT and DCT plots corresponding to impedance during charging. (a-d) DRT plots for charging stages 1-4, (e-h) DCT plots for charging stages 1-4, (i-k) contour plots corresponding to DRT and DCT for charging stages 1-4, (I) Summary of peak changes and mechanisms of DRT and DCT at different charging stages Since DRT is mainly used in fuel cell systems, where reactions are singular and side reactions are few, even when applied to battery systems, it is mostly used to assess the growth of the SEI layer and battery degradation, with DRT peaks showing a relatively simple trend of change (increase/decrease), corresponding to the increase and decrease of impedance R values. Given that the electrode area remains unchanged, it can generally be considered related to the thickening or property changes of the SEI layer. However, in the lithium-oxygen system, the electrode reactions and interfacial processes are more complex, with multiple competing processes, and the active sites of the catalyst are limited. The increase and decrease of DRT peaks not only correspond to changes in reaction Rct but may also be due to one reaction gradually replacing another, becoming the main reaction (or process) on that catalyst.As shown in Figure 4, on a given area of the electrode (corresponding to a given total amount of active sites), when reaction 1 (blue block) transitions to reaction 2 (orange block), various changes in DRT peaks will occur depending on R1/R2 and C1/C2, not necessarily following the trends of ↑↓ and ↗↙. For example, the direct decomposition of Li2O2 or delithiation tends to be visually represented in the DRT plot as the main reaction rather than the one with slow kinetics (even if a reaction is slow, if its proportion is small, it will not be reflected in the EIS). Applied to the charging process of lithium-oxygen batteries, although both Super P carbon black and the catalyst surface can decompose Li2O2, these two processes are competitive; the decomposition reaction on the catalyst surface is much faster than that on carbon black, thus the reaction on the carbon black surface contributes little to the EIS spectrum.As shown in Figure 3, in the early stage of the reaction (stage 1), the overall impedance is small, and the overpotential is not large. Although the decomposition kinetics of Li2O2 slightly increase, the main contribution still comes from the passivation of carbonates. In the early stage of the reaction (stage 2), as the decomposition of organic carbonates between Li2O2 and the catalyst increases, the decomposition of Li2O2 occupies more catalyst sites, becoming the main reaction and the primary source of overpotential. At this time, the properties of the SEI layer also undergo some changes, and the decomposition of lithium carbonate leads to side reactions (such as singlet oxygen, etc.), causing some side reactions in the electrode pores, affecting the diffusion process. In the later stage of the reaction (stage 3), as the decomposition of Li2O2 consumes, its contribution to impedance and overpotential decreases, and the main reaction transitions from Li2O2 decomposition to lithium carbonate decomposition, with the potential gradually increasing, and the SEI layer also begins to decompose, enhancing the overall conductivity of the electrode. In the final stage of the reaction (stage 4), the Li2O2 that can be reached on the catalyst surface and the easily decomposable SEI have all decomposed, leaving behind substances that are difficult to decompose, such as lithium carbonate, which at this point becomes the main source of overpotential due to its slow decomposition kinetics.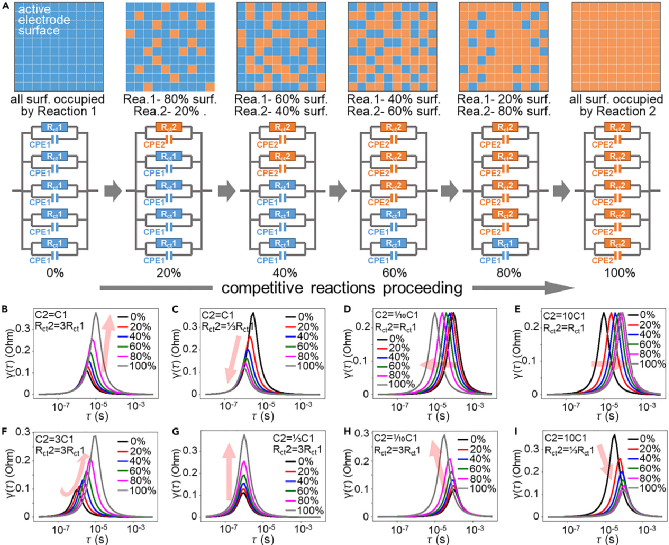 Figure 4 Changes in DRT peaks when one reaction (Rct1//CPE1) is gradually replaced by another reaction (Rct1//CPE1) on a given electrode area. (a) Schematic diagram of one reaction gradually replacing another on a given electrode surface, (b) DRT changes of competitive reactions under different Rct and C conditions 【Conclusion】The authors explored the charging mechanism of lithium-oxygen batteries and the sources of charging overpotential through in-situ impedance and in-situ electrochemical mass spectrometry, utilizing impedance analysis techniques DRT and DCT. This work demonstrates a case of using DRT and DCT for impedance analysis in batteries with multiple competing processes, highlighting the advantages of these techniques in analyzing impedance results, which can help us more intuitively identify the true bottleneck processes in complex systems. Juan Chen, Emanuele Quattrocchi, Francesco Ciucci, Yuhui Chen, Charging processes in lithium-oxygen batteries unraveled through the lens of the distribution of relaxation times, Chem, 2023. https://doi.org/10.1016/j.chempr.2023.04.022
Figure 4 Changes in DRT peaks when one reaction (Rct1//CPE1) is gradually replaced by another reaction (Rct1//CPE1) on a given electrode area. (a) Schematic diagram of one reaction gradually replacing another on a given electrode surface, (b) DRT changes of competitive reactions under different Rct and C conditions 【Conclusion】The authors explored the charging mechanism of lithium-oxygen batteries and the sources of charging overpotential through in-situ impedance and in-situ electrochemical mass spectrometry, utilizing impedance analysis techniques DRT and DCT. This work demonstrates a case of using DRT and DCT for impedance analysis in batteries with multiple competing processes, highlighting the advantages of these techniques in analyzing impedance results, which can help us more intuitively identify the true bottleneck processes in complex systems. Juan Chen, Emanuele Quattrocchi, Francesco Ciucci, Yuhui Chen, Charging processes in lithium-oxygen batteries unraveled through the lens of the distribution of relaxation times, Chem, 2023. https://doi.org/10.1016/j.chempr.2023.04.022
Academician Sun Shigang’s Nature sub-journal: Preparation of highly stable lithium metal anodes through lithium surface modification with fluorinated carboxylic acids
2023-05-24

Solid-state battery expert — Professor Jürgen Janek from the University of Giessen: In-depth analysis of the impact of “pores” on lithium metal anodes in solid-state batteries through in-situ impedance
2023-05-24

AI-assisted battery research | Automation design and practice in battery research in the AI4S era (July 14, 2023, all day)
2023-05-24

First batch of reporting companies and registration units announced: GAC Group Research Institute/Penghui/Jiwan Technology/Tianqi/Chuneng/Betterray/Saiwei/Rongtong (July 13-16, Guangzhou)
2023-05-24

Research progress of biomass-based functional carbon materials in energy conversion and storage
2023-05-24
Chen Hao from Guangdong University of Technology/Chong Yulin from Griffith University/Su Chenliang from Shenzhen University: The efficient synergistic effect of the heterostructured La2O3-Ti3C2Tx promotes high-performance lithium-sulfur batteries
2023-05-24

Academician Ouyang Xiaoping’s team: Structural engineering strategies to promote high stability potassium metal anodes
2023-05-24
He Xiangming’s research group: Wang Li AFM: Three-dimensional built-in interface regulates ion transport and interfacial stability to achieve stable lithium metal anodes
2023-05-23

200 cycles/99.6% efficiency! Dry pre-lithiation aids high-performance silicon-based all-solid-state batteries
2023-05-23
First batch of reporting companies and registration units announced: GAC Group Research Institute/Penghui/Jiwan Technology/Tianqi/Chuneng/Betterray/Saiwei/Rongtong (July 13-16, Guangzhou)
2023-05-23
