Shandong Provincial Medical Association of Ultrasound
Submission Date: 2022-11-01
Corresponding Author:
Li Jie, Email: [email protected].
Abstract:Nano-ultrasound contrast agents possess unique features such as liquid-gas phase transition and acoustic cavitation effects, making them important potential carriers for siRNA delivery. Currently, siRNA loading is mainly achieved through electrostatic attraction, requiring the construction of positively charged contrast agents or the introduction of intermediates like polyethyleneimine, which raises concerns about cationic toxicity. Negative-charged nano-ultrasound contrast agents are free from cationic toxicity and have higher biosafety, but they cannot directly adsorb siRNA. How to achieve siRNA loading using negative-charged nano-ultrasound contrast agents requires further research and standardization. Therefore, this consensus is established to utilize bacteriophage phi29 motor-packaged RNA (3WJ-pRNA) genetic modification technology to prepare gene particles/nano-ultrasound contrast agent nanocomposites, verifying their good stability, biosafety, contrast ultrasound imaging capability, and strong siRNA delivery ability, laying the foundation for standardizing the loading and delivery of siRNA using negative-charged nano-ultrasound contrast agents.
Keywords: Nano-ultrasound contrast agents, Genetic modification technology, Nano-composite, siRNA delivery, Ultrasound examination
Consensus on the application of RNA genetic modification in carrying and delivering siRNA with negative-
charged nano-ultrasound contrast
agent
Shandong Provincial Medical Association of Ultrasound
RNA-based gene therapy has advantages such as simple design and the ability to target various pathogenic targets, making it a promising effective treatment strategy for refractory diseases, including malignant tumors [1]. However, the delivery of siRNA into cells faces two major challenges: one is the stability issue when exposed to blood, which can lead to immunogenicity; the other is that negatively charged siRNA cannot cross the membrane into the cells by itself [2]. Therefore, the delivery of siRNA requires various nano-carriers. Common carriers mainly include viral vectors and liposomal carriers, which, although widely used in basic research, are not suitable for direct clinical use. The application of delivery carriers has dual standards of safety and effectiveness. Viral vectors bring uncertainties in safety, while liposomal carriers face efficiency challenges. Nano-ultrasound contrast agents are a new type of nano-carrier, typically with particle sizes ranging from 200 to 500 nm, which not only have ultrasound imaging capabilities but also serve as potential drug or gene delivery carriers [3-4]. Since siRNA carries a negative charge, most nano-carriers, including ultrasound contrast agents, currently load siRNA through electrostatic attraction, which requires constructing nano-carriers with positively charged surfaces or introducing positively charged intermediates like polyethyleneimine, which has the drawback of cationic toxicity [5-7]. In contrast, negatively charged nano-ultrasound contrast agents do not have cationic toxicity issues and possess higher biosafety and clinical application value. Therefore, how to load siRNA onto negatively charged nano-ultrasound contrast agents requires further research and standardization. This consensus aims to standardize issues related to the loading and delivery of siRNA using negatively charged nano-ultrasound contrast agents.1 Preparation and Identification of Gene Nanoparticles Based on Bacteriophage phi29 Motor-Packaged RNA(three-way junction of bacteri
ophage phi29 motor packaging RNA, 3WJ-pRNA) modification
3WJ-pRNA can self-fold into a three-dimensional structure with three angles based on base pairing principles, with the advantage that the three side chains can be modified at their ends to achieve different functions. Its core structure consists of nucleic acid sequences, which are easy to modify and synthesize, and are non-toxic, making it a suitable carrier for siRNA loading and RNA/DNA aptamer modification [8-10]. Cholesterol-modified 3WJ-pRNA gene particles can anchor to lipid vesicles or exosomes, which have a core-shell structure, providing a new idea for the modification of nano-ultrasound contrast agents with 3WJ-pRNA and subsequently loading siRNA.
1.1 Preparation of 3WJ-pRNA Gene Nanoparticles3WJ-pRNA has a fixed core sequence [13] (Table 1). Four RNA single strands were designed and synthesized based on the core sequence (Table 2), which are Chol-modified aWJ side chain (aWJ-Chol, Strand 1, anchoring module), A10-3.2 modified bWJ side chain (bWJ-A10-3.2, Strand 2, targeting module), siCAT-1 modified cWJ side chain (cWJ-siCAT-1, Strand 3, therapeutic module), and the antisense strand of the therapeutic module (siCAT-1 antisense, Strand 4). The design of the modification strategy is shown in Figure 1. The four RNA single strands were dissolved in diethyl carbonate to obtain a concentration of 50-100 μmol stock solution. The four RNA single strands in equimolar proportions were mixed in DEPC water and annealed in the annealing buffer, followed by programmed cooling [12] to obtain the gene nanoparticles.
Table 1 3WJ-pRNA Core Sequence
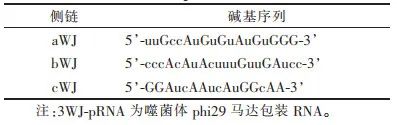
Table 2 Sequences of Four Single Strands of Gene Nanoparticles Based on 3WJ-pRNA Modification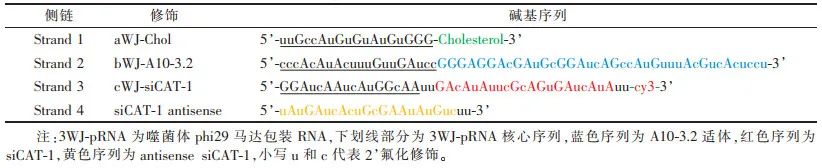
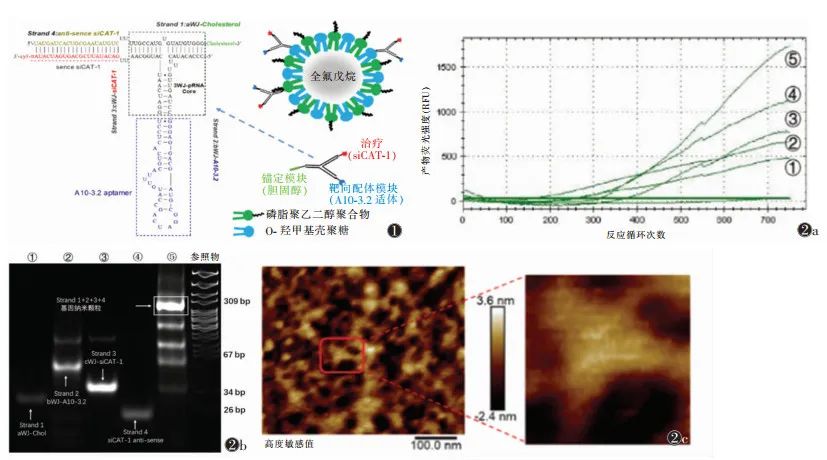 Figure 1 Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposite Model Based on Bacteriophage phi29 Motor-Packaged RNA (3WJ-pRNA) Figure 2 Characterization of 3WJ-pRNA Gene Nanoparticles Note: Figure 2a shows SYBR Green I real-time fluorescence curve detection of synthesis efficiency; Figure 2b shows non-denaturing gel electrophoresis detection of molecular weight; Figure 2c shows atomic force microscopy detection of morphology. ①~④ represent RNA single strands Strand 1~Strand 4, ⑤ represents the product gene nanoparticles mixed from Strand 1~Strand 41.2 Identification of 3WJ-pRNA Gene Nanoparticles The identification of gene nanoparticles mainly includes synthesis efficiency, molecular weight, and morphology. SYBR Green I can bind to double-stranded nucleic acids and emit green fluorescence, and the fluorescence intensity is related to the amount of double-stranded nucleic acids. Therefore, SYBR Green I real-time fluorescence can be used to detect synthesis efficiency, and the fluorescence curve detected by real-time quantitative PCR indicates that the four single strands of RNA gradually form double-stranded 3WJ-pRNA gene nanoparticles (Figure 2a). 8% non-denaturing gel electrophoresis is used to detect the molecular weight of the synthesized product (Figure 2b). Atomic force microscopy is the most intuitive method to verify the morphology of gene nanoparticles, and the synthesized product exhibits a cloverleaf structure, consistent with the 3WJ-pRNA structure (Figure 2c). Through the above three detection methods, it is confirmed that the 3WJ-pRNA gene nanoparticles were successfully synthesized.
Figure 1 Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposite Model Based on Bacteriophage phi29 Motor-Packaged RNA (3WJ-pRNA) Figure 2 Characterization of 3WJ-pRNA Gene Nanoparticles Note: Figure 2a shows SYBR Green I real-time fluorescence curve detection of synthesis efficiency; Figure 2b shows non-denaturing gel electrophoresis detection of molecular weight; Figure 2c shows atomic force microscopy detection of morphology. ①~④ represent RNA single strands Strand 1~Strand 4, ⑤ represents the product gene nanoparticles mixed from Strand 1~Strand 41.2 Identification of 3WJ-pRNA Gene Nanoparticles The identification of gene nanoparticles mainly includes synthesis efficiency, molecular weight, and morphology. SYBR Green I can bind to double-stranded nucleic acids and emit green fluorescence, and the fluorescence intensity is related to the amount of double-stranded nucleic acids. Therefore, SYBR Green I real-time fluorescence can be used to detect synthesis efficiency, and the fluorescence curve detected by real-time quantitative PCR indicates that the four single strands of RNA gradually form double-stranded 3WJ-pRNA gene nanoparticles (Figure 2a). 8% non-denaturing gel electrophoresis is used to detect the molecular weight of the synthesized product (Figure 2b). Atomic force microscopy is the most intuitive method to verify the morphology of gene nanoparticles, and the synthesized product exhibits a cloverleaf structure, consistent with the 3WJ-pRNA structure (Figure 2c). Through the above three detection methods, it is confirmed that the 3WJ-pRNA gene nanoparticles were successfully synthesized.
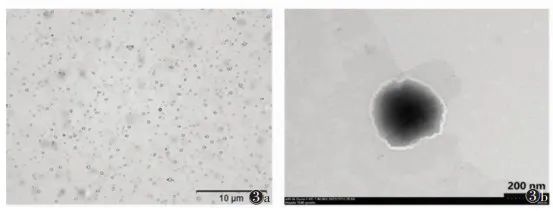
2 Preparation and Performance Detection of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites Based on 3WJ-pRNA Modification The selection of shell materials for nano-ultrasound contrast agents needs to consider two factors: the ability to anchor cholesterol-modified 3WJ-pRNA gene particles and high biosafety. Therefore, this consensus uses low-toxicity carboxymethyl chitosan (O-CMC) and lipids (DSPE-PEG) to jointly form the shell structure of nano-ultrasound contrast agents, both of which have good biocompatibility, and O-CMC also has certain anti-tumor effects [14-16].2.1 Preparation of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites The preparation of nano-ultrasound contrast agents refers to relevant literature [17]. Perfluorohexane, Tween 20, and lipid DSPE-PEG were dispersed in deionized water to obtain a suspension through high-speed homogenization; under high-speed mechanical stirring conditions, O-CMC solution was added dropwise to the above suspension to obtain an emulsion; the emulsion was allowed to stand at room temperature, low-speed centrifuged, and the upper liquid was filtered through a 0.45 μm filter membrane to obtain nano-ultrasound contrast agent O-CMC/DSPE-PEG-NDs. Then, the 3WJ-pRNA gene nanoparticles were co-cultured with O-CMC/DSPE-PEG-NDs nano-ultrasound contrast agents in a certain proportion to obtain 3WJ-pRNA modified gene particles/nano-ultrasound contrast agent-nanocomposites. 2.2 Characterization of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites The characterization of gene particles/nano-ultrasound contrast agent-nanocomposites mainly includes morphological structure, particle size, and potential detection. Morphology can be observed using optical microscopy and transmission electron microscopy, with optical microscopy (using a 100x oil immersion lens, total magnification ≥ 1,000 times) showing that the nano-composites are uniformly sized circular structures, evenly dispersed without aggregation (Figure 3a); transmission electron microscopy can reveal the core-shell structure of the composite (Figure 3b). The particle size and surface potential were analyzed using a nano-laser particle size and Zeta potential analyzer [18], with an average particle size of 198 nm, a polydispersity index of 0.306, and a surface potential of -12.98 mV. The combination of 3WJ-pRNA gene nanoparticles and O-CMC/DSPE-PEG-NDs can be verified using fluorescence colocalization methods, labeling gene nanoparticles as green and ultrasound nano-droplets as red; if fluorescence overlaps to yellow, it indicates that the two are combined.
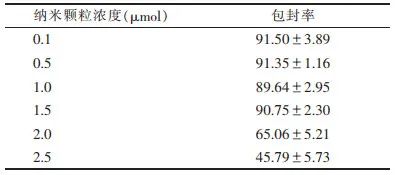
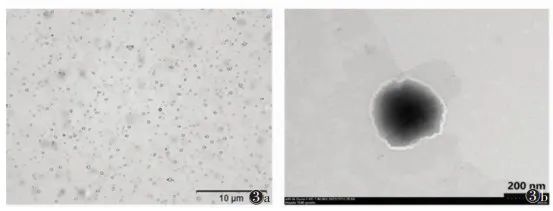 Note: Figure 3a is an optical microscope image, Figure 3b is a transmission electron microscope image Figure 3 Characterization of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites Modified by Bacteriophage phi29 Motor-Packaged RNA (3WJ-pRNA)2.3 Detection of Gene Loading Efficiency of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites The gene loading efficiency of the nano-composite was measured using a Nanodrop 2000 micro-spectrophotometer. To obtain the optimal loading efficiency, the concentration range of gene nanoparticles was set between 0.1 and 2.5 μmol. When the concentration of gene nanoparticles > 1.5 μmol, the gene encapsulation rate showed a significant downward trend (Table 3). Therefore, the gene loading efficiency is optimal when the concentration of gene nanoparticles is 1.5 μmol. Additionally, gel retardation experiments can further validate the gene loading efficiency, as the genes carried by the nano-composite will not be displayed in the gel image during electrophoresis.Table 3 Gene Encapsulation Rate Detection of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites (%, x±s)
Note: Figure 3a is an optical microscope image, Figure 3b is a transmission electron microscope image Figure 3 Characterization of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites Modified by Bacteriophage phi29 Motor-Packaged RNA (3WJ-pRNA)2.3 Detection of Gene Loading Efficiency of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites The gene loading efficiency of the nano-composite was measured using a Nanodrop 2000 micro-spectrophotometer. To obtain the optimal loading efficiency, the concentration range of gene nanoparticles was set between 0.1 and 2.5 μmol. When the concentration of gene nanoparticles > 1.5 μmol, the gene encapsulation rate showed a significant downward trend (Table 3). Therefore, the gene loading efficiency is optimal when the concentration of gene nanoparticles is 1.5 μmol. Additionally, gel retardation experiments can further validate the gene loading efficiency, as the genes carried by the nano-composite will not be displayed in the gel image during electrophoresis.Table 3 Gene Encapsulation Rate Detection of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites (%, x±s)
2.4 Ultrasound Imaging Ability and Stability Detection of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites An ultrasound diagnostic instrument (Logiq E9) was used to detect the ultrasound imaging level and stability of the nano-composite [19]. In vitro ultrasound imaging can be conducted using 2% agarose or plastic droppers, showing both grayscale images and enhanced scanning images (Figure 4), and observed for at least 10 minutes to assess the stability of the nano-composite. The nano-composite achieved good contrast-enhanced ultrasound imaging effects and could remain stable for at least 10 minutes, with the contrast-enhanced ultrasound intensity at 0 minutes being (38.32±2.14)dB, and at 10 minutes being (20.83±2.95)dB, indicating that the nano-composite has good stability and strong ultrasound imaging capability.
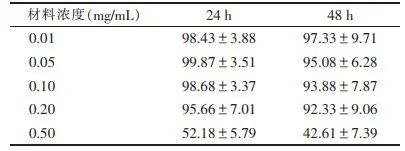
 Note: Deionized water as a negative control Figure 4 Grayscale Ultrasound and Contrast-Enhanced Ultrasound Images of the Nano-Composite2.5 Evaluation of Biological Safety of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites The toxicity of the nano-composite to cells can reflect its biosafety. The preparation material mixture of the nano-composite (including O-CMC, DSPE-PEG, siRNA) was added to the cell culture medium at concentrations of 0.01, 0.05, 0.10, 0.20, and 0.50 mg/mL, and the optical absorbance was detected at a wavelength of 450 nm using a cell counting kit (CCK-8) method. The results showed that when the usage concentration was below (≤0.20 mg/mL), cell viability was > 95%, indicating that the biosafety of the nano-composite is relatively high (Table 4).Table 4 Biological Safety Evaluation of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites (%, x±s)
Note: Deionized water as a negative control Figure 4 Grayscale Ultrasound and Contrast-Enhanced Ultrasound Images of the Nano-Composite2.5 Evaluation of Biological Safety of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites The toxicity of the nano-composite to cells can reflect its biosafety. The preparation material mixture of the nano-composite (including O-CMC, DSPE-PEG, siRNA) was added to the cell culture medium at concentrations of 0.01, 0.05, 0.10, 0.20, and 0.50 mg/mL, and the optical absorbance was detected at a wavelength of 450 nm using a cell counting kit (CCK-8) method. The results showed that when the usage concentration was below (≤0.20 mg/mL), cell viability was > 95%, indicating that the biosafety of the nano-composite is relatively high (Table 4).Table 4 Biological Safety Evaluation of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites (%, x±s)
3 siRNA Targeted Delivery Capability of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites
3.1 Targeting Ability of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites The nano-composite was co-cultured with prostate-specific membrane antigen (PSMA)-expressing 22RV1 cells, and unbound nano-composites were washed away with phosphate-buffered saline (PBS), and the targeting ability was analyzed using flow cytometry [20]. Meanwhile, PSMA low-expressing PC-3 cells and non-targeted nano-composites were used as negative controls. The results showed that compared to non-targeted nano-composites, targeted nano-composites had a stronger binding to 22RV1 cells (92.30% vs. 3.69%); the binding of targeted nano-composites to 22RV1 cells was significantly higher than that to PC-3 cells (92.30% vs. 7.52%). This indicates that the nano-composite can effectively target and bind to 22RV1 cells, and the targeting ability is positively correlated with the PSMA expression level in the cells. 3.2 siRNA Delivery Capability of Gene Particles/Nano-Ultrasound Contrast Agent-Nanocomposites The nano-composite was co-cultured with 22RV1 cells, and after washing unbound nano-composites with PBS, the cells were further cultured for 24 hours, and total RNA was extracted to detect CAT-1 mRNA expression levels through RT-PCR. The results showed that the nano-composite combined with ultrasound irradiation significantly downregulated CAT-1 mRNA expression levels, and the downregulation capability was significantly stronger than that of non-ultrasound irradiated nano-composites, non-targeted nano-composites combined with ultrasound irradiation, and liposomal siRNA delivery (conventional transfection siCAT-1+lipofectamin) (Table 5). This indicates that the nano-composite can effectively deliver siRNA to tumor cells.Table 5 siRNA Delivery Capability of the Nano-Composite (%, x±s)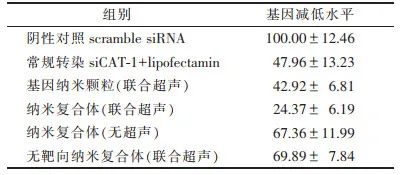
In summary, this consensus adopts the 3WJ-pRNA modification technology to prepare gene particles/nano-ultrasound contrast agent-nanocomposites, which have high biosafety and good contrast-enhanced ultrasound imaging levels, capable of effectively carrying siRNA and targeting delivery to tumor cells. This gene particles/nano-ultrasound contrast agent-nanocomposite is a novel gene delivery system that can achieve effective siRNA delivery under ultrasound assistance, providing a new approach for tumor gene diagnosis and treatment, and laying the foundation for standardizing the loading and delivery of siRNA using negatively charged nano-ultrasound contrast agents.Core Authors of the Consensus:Li Jie (Department of Ultrasound, Qilu Hospital of Shandong University), Dong Lei (Department of Ultrasound, 960 Hospital of Joint Logistics Support Force of PLA), Wang Zhibin (Department of Ultrasound, Affiliated Hospital of Qingdao University), Jiang Zhongqiang (Department of Ultrasound, Affiliated Children’s Hospital of Shandong University), Zhao Cheng (Department of Ultrasound, Affiliated Hospital of Qingdao University), Wu Mei (Department of Ultrasound, Second Hospital of Shandong University), Wang Bei (Department of Ultrasound, First Affiliated Hospital of Shandong First Medical University).Acknowledgements: In the process of writing this consensus, 56 experts provided valuable revision suggestions, and we sincerely thank them.
References:
| [1] |
ROSSI J J, ROSSI D J. siRNA drugs: here to stay[J]. Mol Ther, 2021, 29(2): 431-432. DOI:10.1016/j.ymthe.2021.01.015 |
| [2] |
CHARBE N B, AMNERKAR N D, RAMESH B, et al. Small interfering RNA for cancer treatment: overcoming hurdles in delivery[J]. Acta Pharm Sin B, 2020, 10(11): 2075-2109. DO.10.1016/j.apsb.2020.10.005 |
| [3] |
CHOWDHURY S M, ABOU-ELKACEM L, LEE T, et al. Ultrasound and microbubble mediated therapeutic delivery: Underlying mechanisms and future outlook[J]. J Control Release, 2020, 326: 75-90. DOI:10.1016/j.jconrel.2020.06.008 |
| [4] |
LI J, XI A, QIAO H, et al. Ultrasound-mediated diagnostic imaging and advanced treatment with multifunctional micro/nanobubbles[J]. Cancer Lett, 2020, 475: 92-98. DOI:10.1016/j.canlet.2020.01.028 |
| [5] |
WEISS M, FAN J, CLAUDEL M, et al. Density of surface charge is a more predictive factor of the toxicity of cationic carbon nanoparticles than zeta potential[J]. J Nanobiotechnology, 2021, 19(1): 5. DOI:10.1186/s12951-020-00747-7 |
| [6] |
TAI W. Chemical modulation of siRNA lipophilicity for efficient delivery[J]. J Control Release, 2019, 307: 98-107. |
| [7] |
LIU L, LIU Y, XU B, et al. Negative regulation of cationic nanoparticle-induced inflammatory toxicity through the increased production of prostaglandin E2 via mitochondrial DNA-activated Ly6C(+) monocytes[J]. Theranostics, 2018, 8(11): 3138-3152. DOI:10.7150/thno.21693 |
| [8] |
JASINSKI D L, BINZEL D W, GUO P. One-pot production of RNA nanoparticles via automated processing and selfassembly[J]. ACS Nano, 2019, 13(4): 4603-4612. DOI:10.1021/acsnano.9b00649 |
| [9] |
PI F, BINZEL D W, LEE T J, et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression[J]. Nature Nanotechnology, 2018, 13(1): 82-89. DOI:10.1038/s41565-017-0012-z |
| [10] |
GUO S, PIAO X, LI H, et al. Methods for construction and characterization of simple or special multifunctional RNA nanoparticles based on the 3WJ of phi29 DNA packaging motor[J]. Methods, 2018, 143: 121-133. DOI:10.1016/j.ymeth.2018.02.025 |
| [11] |
LI Z, WANG H, YIN H, et al. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression[J]. Sci Rep, 2018, 8(1): 14644. DOI:10.1038/s41598-018-32953-7 |
| [12] |
HARASZTI R A, MILLER R, DIDIOT M C, et al. Optimized cholesterol-siRNA chemistry improves productive loading onto extracellular vesicles[J]. Mol Ther, 2018, 26(8): 1973-1982. DOI:10.1016/j.ymthe.2018.05.024 |
| [13] |
SHU D, SHU Y, HAQUE F, et al. Thermodynamically stable RNA three-way junction for constructing multifunctional nanoparticles for delivery of therapeutics[J]. Nat Nanotechnol, 2011, 6(10): 658-667. DOI:10.1038/nnano.2011.105 |
| [14] |
DONG X D, YU J, MENG F Q, et al. Antitumor effects of seleno-short-chain chitosan (SSCC) against human gastric cancer BGC-823 cells[J]. Cytotechnology, 2019, 71(6): 1095-1108. DOI:10.1007/s10616-019-00347-w |
| [15] |
CHI J, JIANG Z, QIAO J, et al. Antitumor evaluation of carboxymethyl chitosan based norcantharidin conjugates against gastric cancer as novel polymer therapeutics[J]. Int J Biol Macromol, 2019, 136: 1-12. DOI:10.1016/j.ijbiomac.2019.05.216 |
| [16] |
WANG W, XUE C, MAO X. Chitosan: structural modification, biological activity and application[J]. Int J Biol Macromol, 2020, 164: 4532-4546. DOI:10.1016/j.ijbiomac.2020.09.042 |
| [17] |
MENG D, GUO L, SHI D, et al. Charge-conversion and ultrasound-responsive O-carboxymethyl chitosan nanodroplets for controlled drug delivery[J]. Nanomedicine (Lond), 2019, 14(19): 2549-2565. DOI:10.2217/nnm-2019-0217 |
| [18] |
WANG X, SHANG M, SUN X, et al. Dual-responsive nanodroplets combined with ultrasound-targeted microbubble destruction suppress tumor growth and metastasis via autophagy blockade[J]. J Control Release, 2022, 343: 66-77. DOI:10.1016/j.jconrel.2022.01.009 |
| [19] |
SHANG M, SUN X, GUO L, et al. pH-and ultrasoundresponsive paclitaxel-loaded carboxymethyl chitosan nanodroplets for combined imaging and synergistic chemoradiotherapy[J]. Int J Nanomedicine, 2020, 15: 537-552. DOI:10.2147/IJN.S233669 |
| [20] |
GUO L, SHI D, MENG D, et al. New FH peptide-modified ultrasonic nanobubbles for delivery of doxorubicin to cancer-associated fibroblasts[J]. Nanomedicine (Lond), 2019, 14(22): 2957-2971. DOI:10.2217/nnm-2019-0302 |