iNature
Due to the non-invasive remote driving and drug loading capabilities of magnetic nanorobots, there are broad application prospects in thrombolytic therapy. Although nanorobots smaller than 100 nanometers are suitable for microvascular systems, their propulsion performance is severely affected due to limited response to magnetic fields.
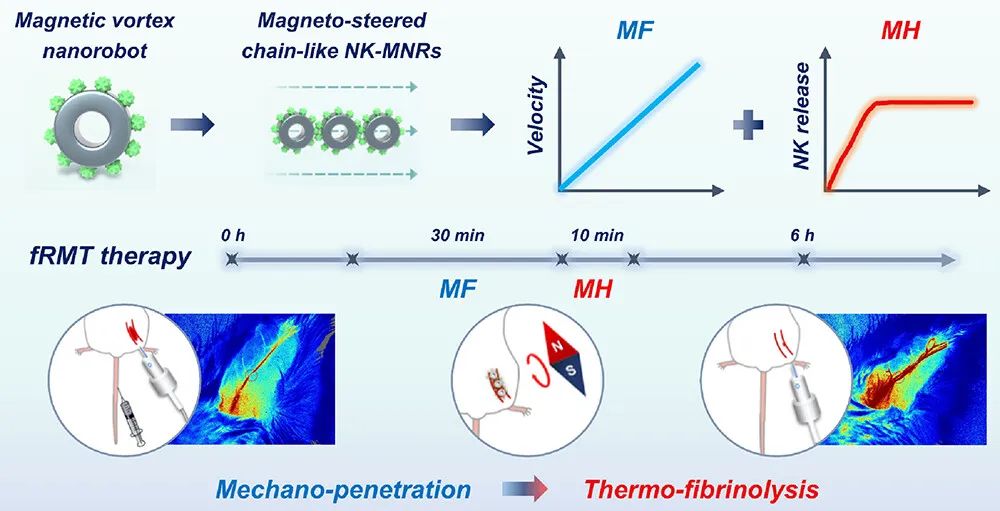
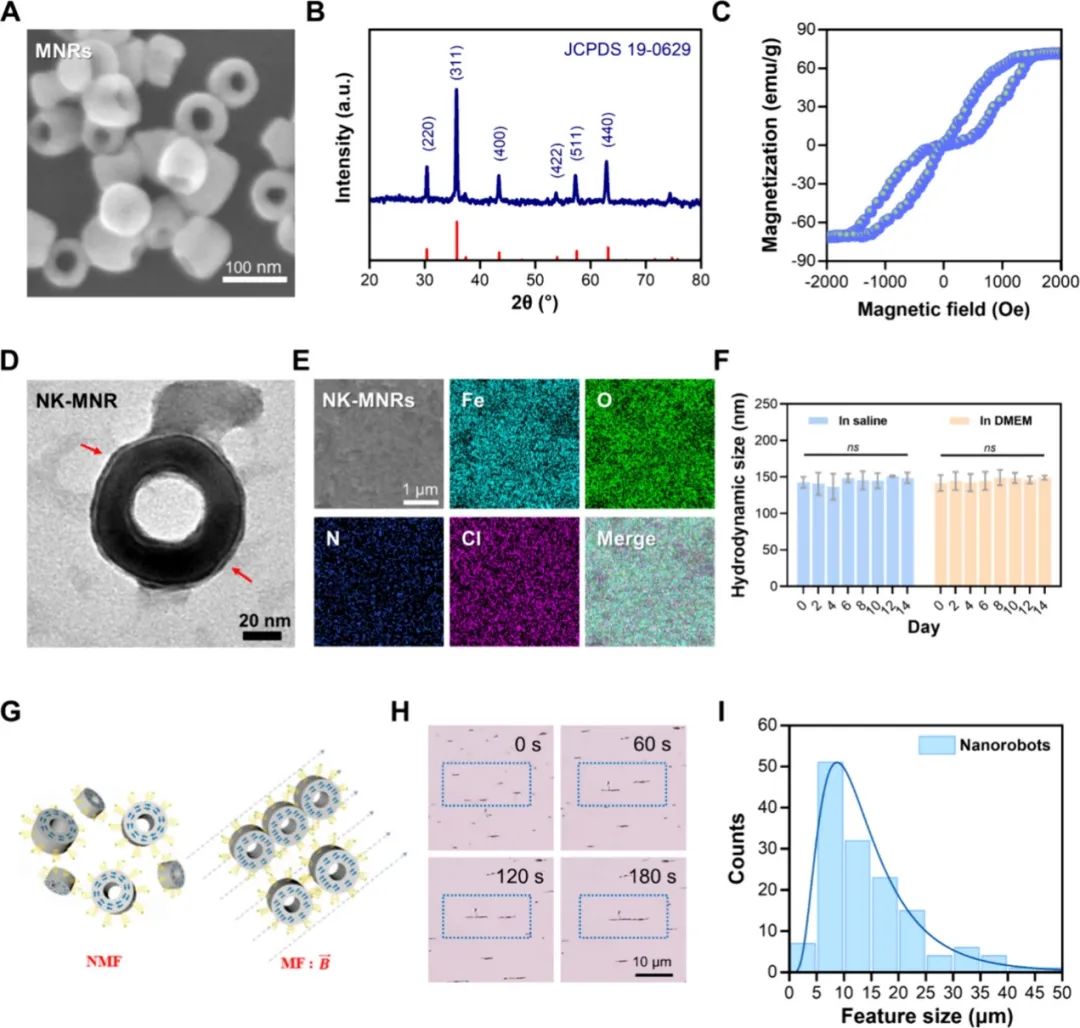
On October 18, 2024, Cheng Yu and He Bin from Tongji University, along with Fan Haiming from Northwest University, jointly communicated a research paper titled “Dual Frequency-Regulated Magnetic Vortex Nanorobots Empower Nattokinase for Focalized Microvascular Thrombolysis” published online in ACS Nano. This study demonstrates a magnetic vortex nanorobot (NK-MNR) loaded with nattokinase, with an average size of approximately 70nm, exhibiting high saturation magnetization, capable of mechanical propulsion and thermal-responsive thrombolysis under dual-frequency magnetic fields.
The nanorobots are stable in suspension and are magnetically manipulated to assemble into chain-like NK-MNRs, mechanically disrupting and penetrating clots through low-frequency rotating magnetic fields. The magnetic hyperthermia triggered by high-frequency alternating magnetic fields synergistically enhances the thermally induced release of NK and fibrinolysis. Under the dual magnetic energy conversion regulation in the dual-frequency regulated magnetic thrombolysis (fRMT) strategy, the nanorobots achieved an 81.0% vascular recanalization rate in thrombotic mice. In summary, nanorobots with special magnetic vortex characteristics and multi-mode control represent an effective in vivo focused microvascular thrombolysis nanoplatform.


—END—
The content is original from 【iNature】,
Reprint must specify the source from 【iNature】
Add WeChat Group
iNature gathers 40,000 researchers and doctors in life sciences. We have established 80 comprehensive groups (16 PI groups and 64 PhD groups), and also formed specialized groups for relevant fields (plants, immunology, cells, microbiology, gene editing, neuroscience, chemistry, physics, cardiovascular, oncology, etc.). Note: Please specify when joining the group (format: school + major + name; if you are a PI/professor, please indicate that you are a PI/professor, otherwise it will be assumed you are a PhD student, thank you).You can first add the editor’s WeChat ID (love_iNature), or long press the QR code to add the editor, and then join the relevant group. Serious inquiries only.


Submission, cooperation, and reprint authorization matters
Please contact WeChat ID:13701829856 or email:[email protected]
If you find this article interesting, please click here!