[OL]Ni Catalyzed C-N Coupling of Brominated Heteroaromatic Compounds
Introduction
Constructing C-N bonds is generally more challenging than constructing C-C bonds. After all, the Buchwald-Hartwig coupling is not effective for all substrates, especially for certain heterocycles and substrates containing active hydrogens, which can easily complex with the catalyst and lead to no reaction. In contrast, usingSuzuki coupling to construct C-C bonds typically allows active hydrogens to not interfere with the reaction process. Therefore, in terms of general applicability,Suzuki coupling is more versatile thanBuchwald coupling.

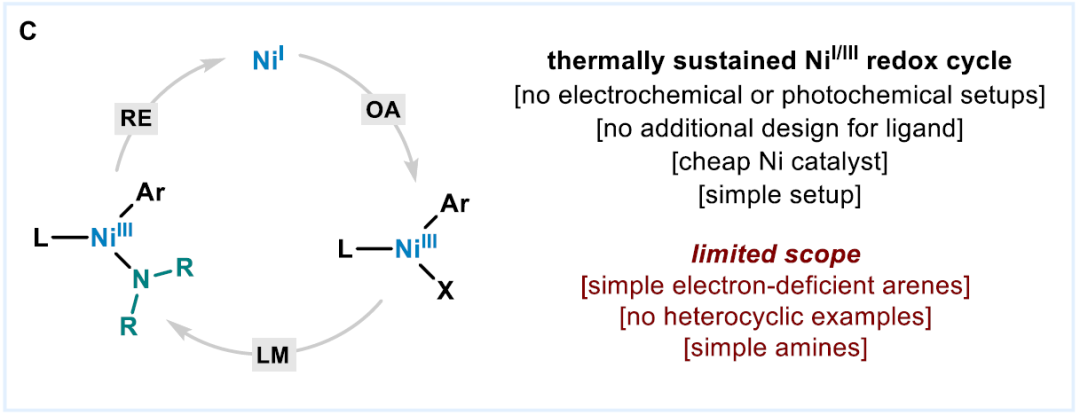
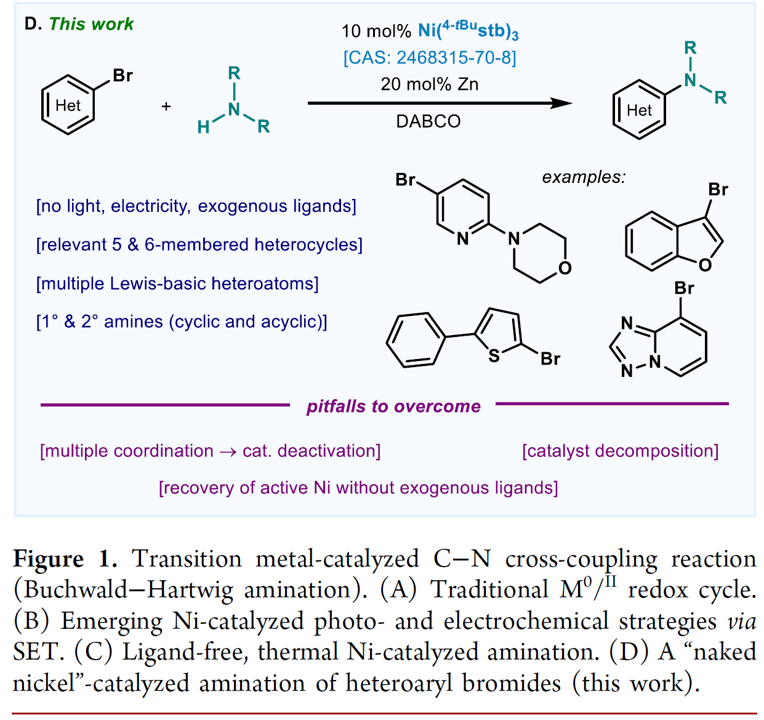
Optimization
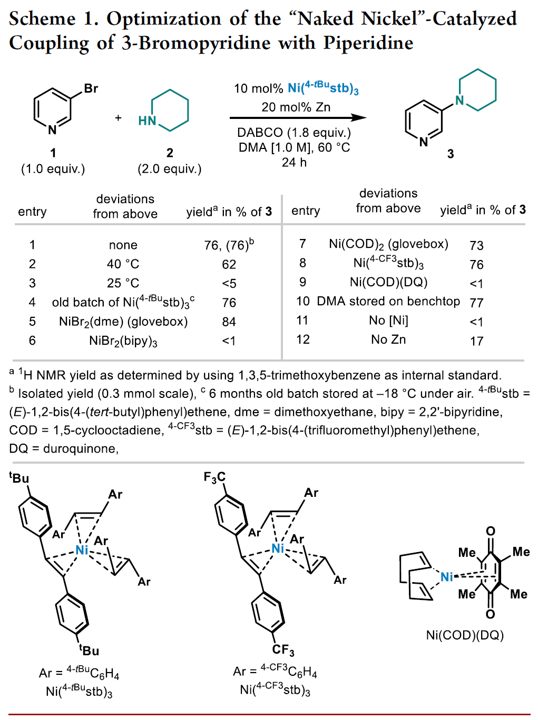

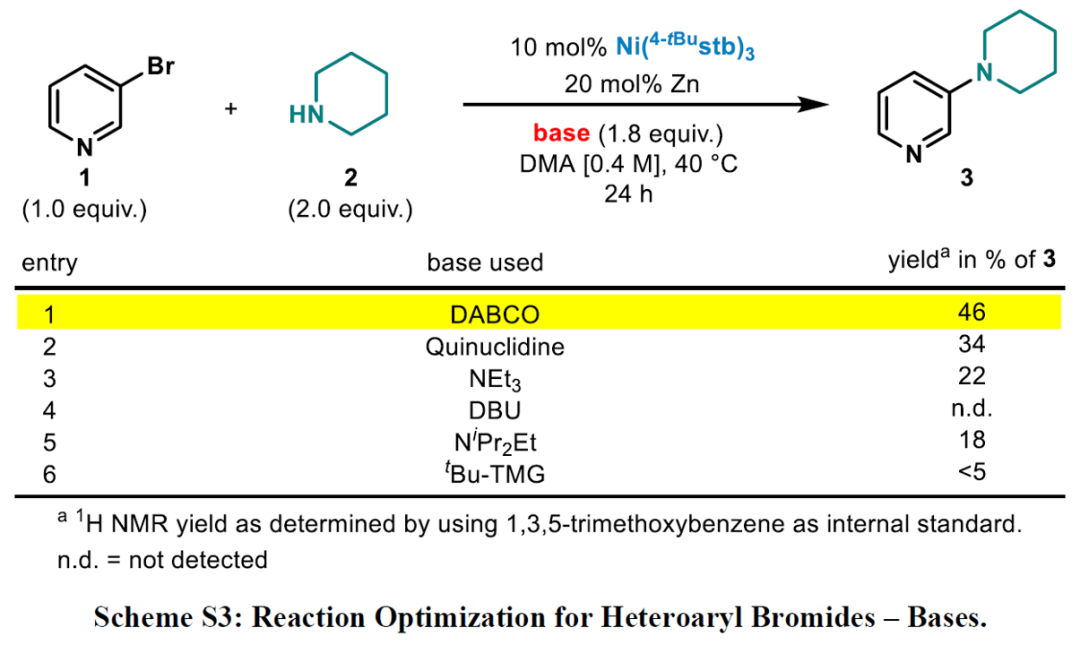
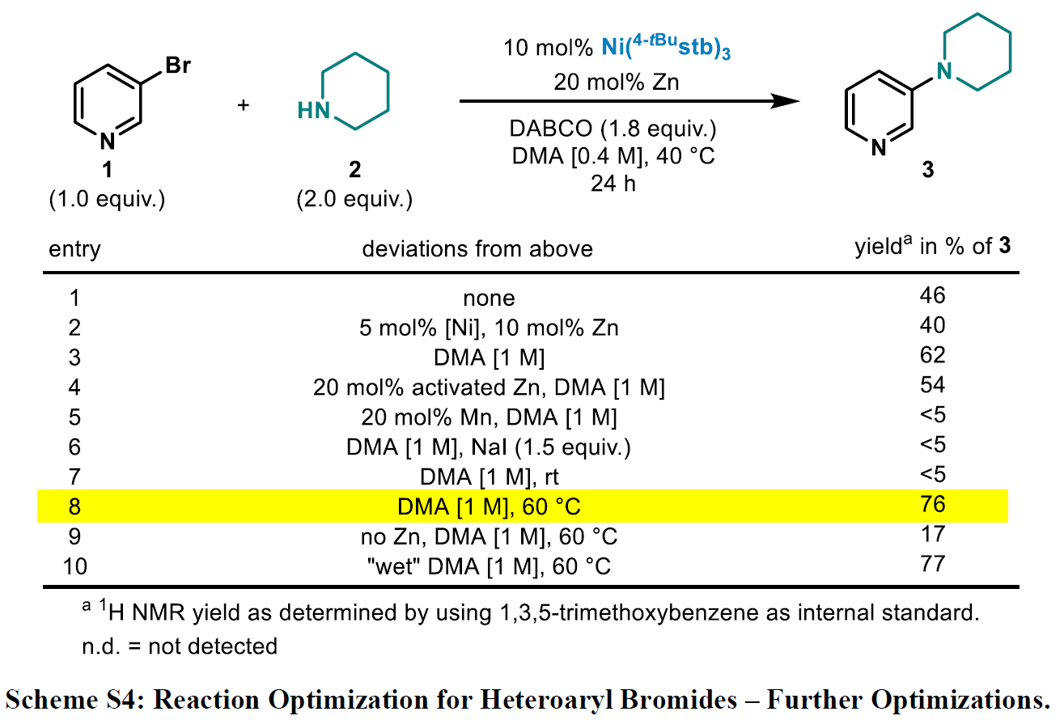
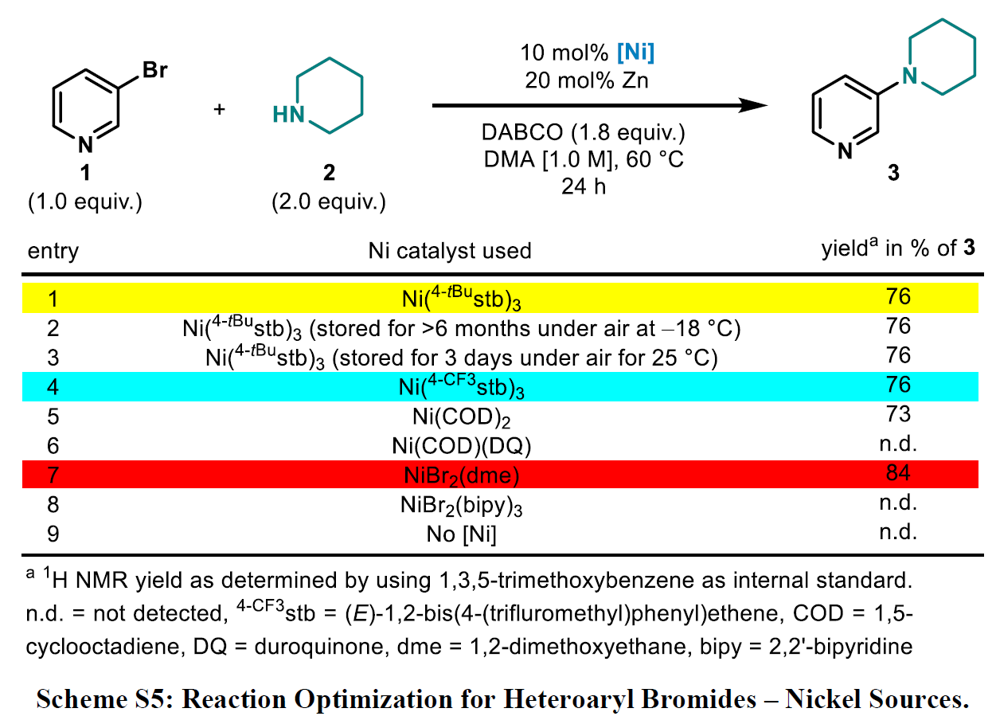
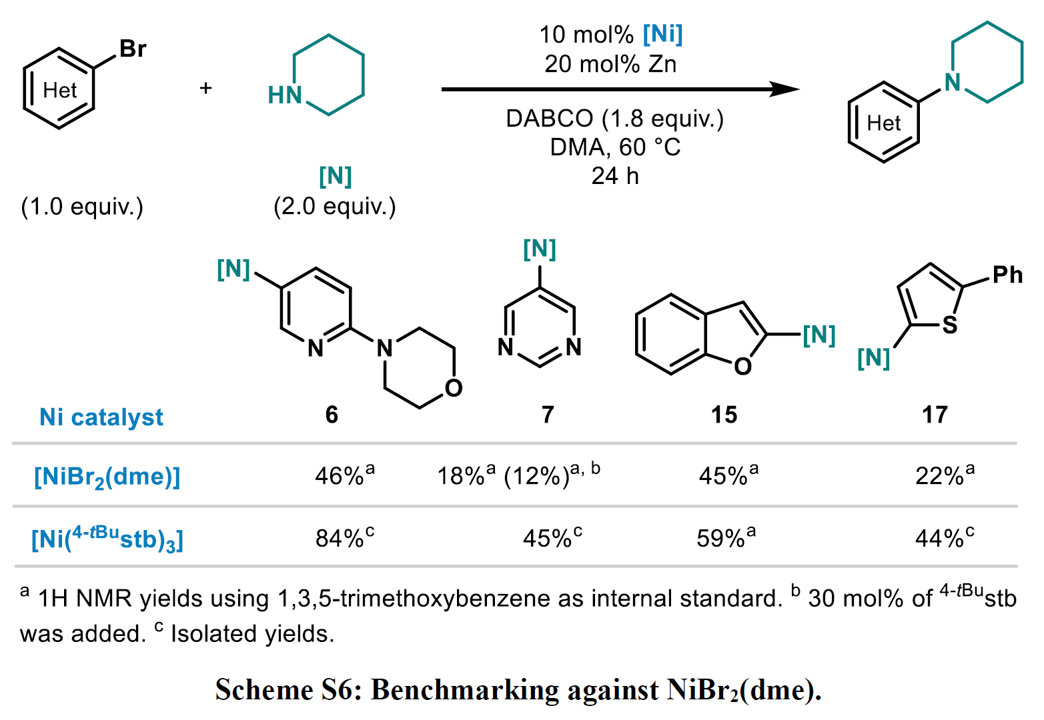

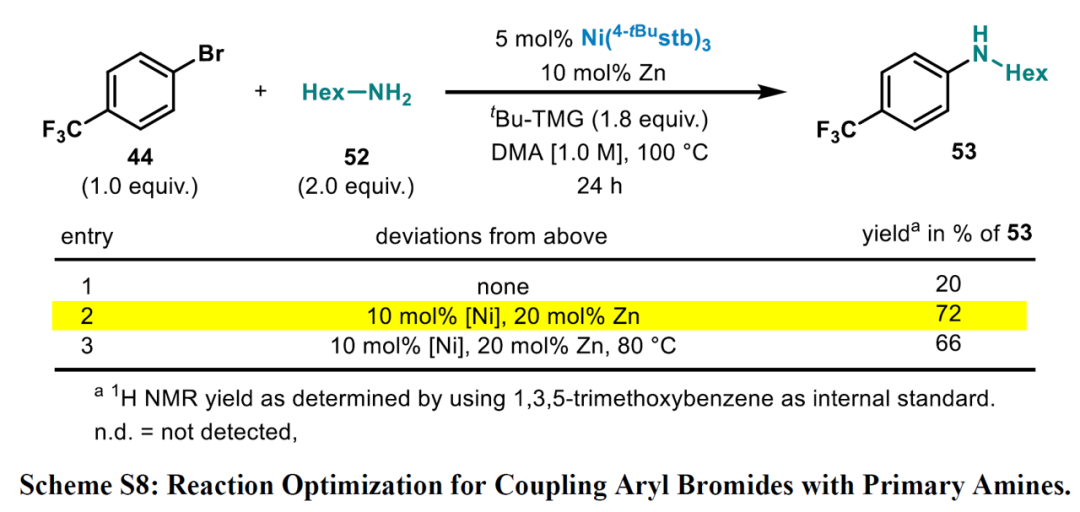
Expansion
Procedure Steps

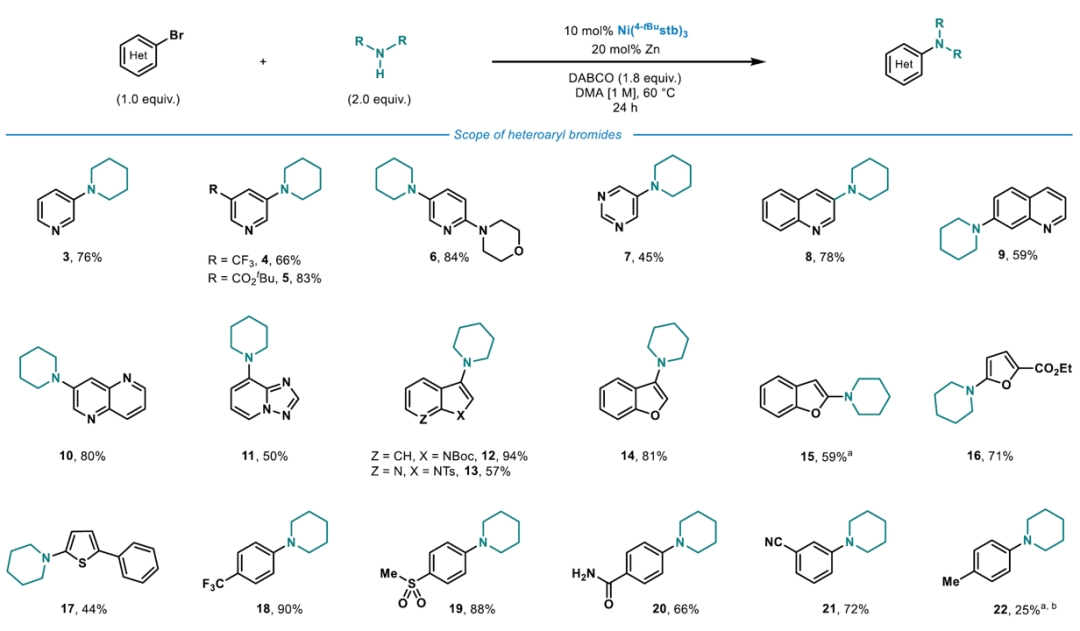
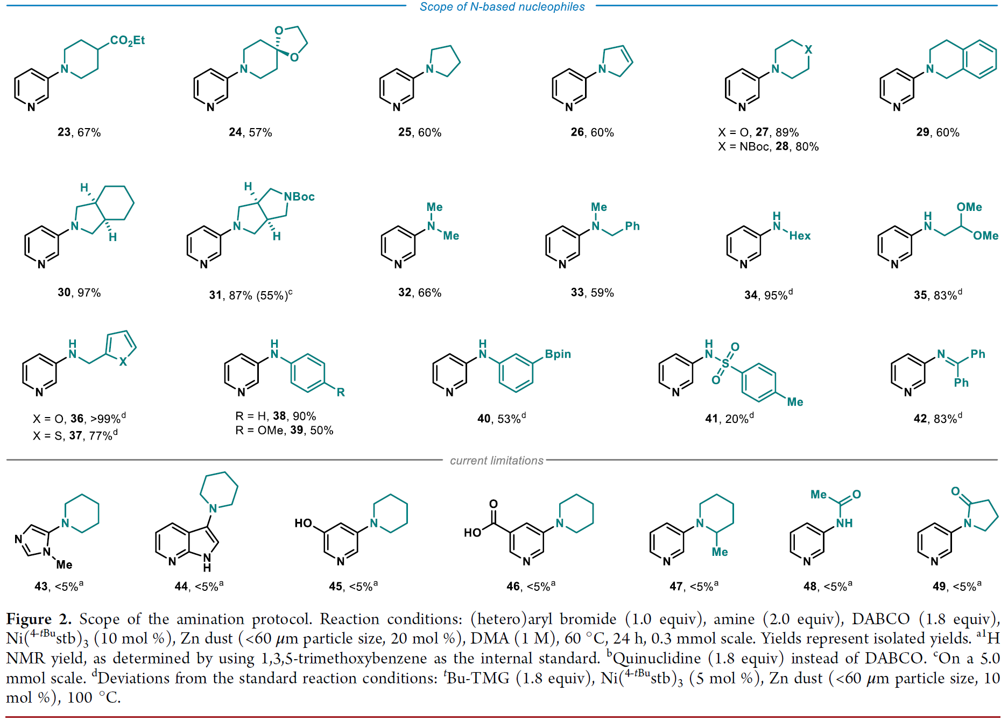
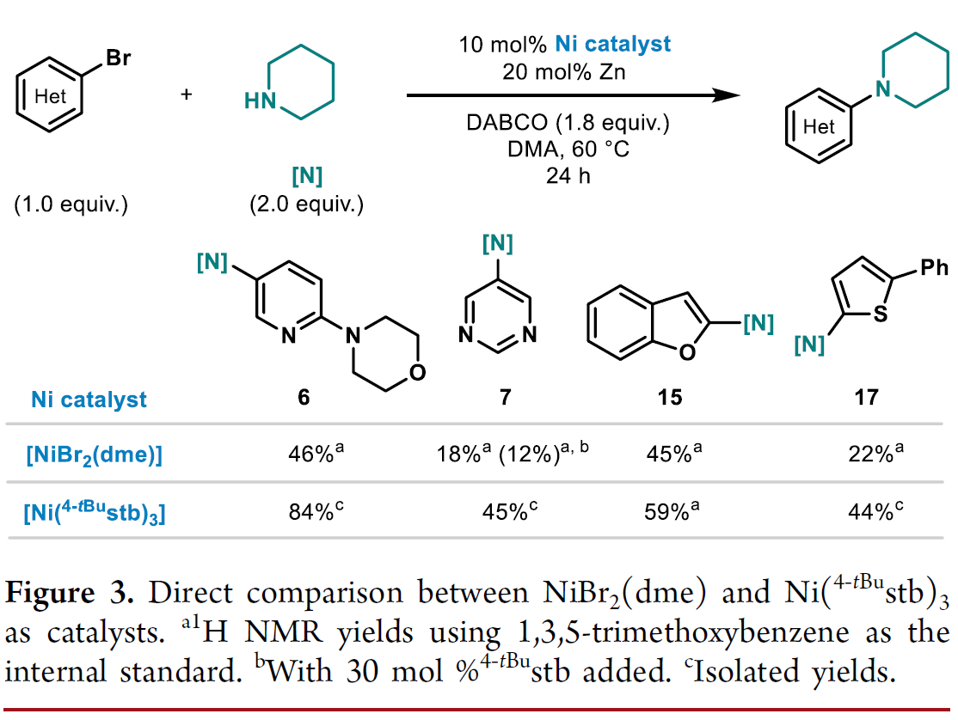
A 12-mL screwcap vial equipped with a stirring bar was dried under vacuum using a heat gun set to 550 °C, followed by three cycles of vacuum/argon. The vial was charged with Ni(4-tBustb)3 (10 mol%), Zn dust (< 60 μm, 20 mol%), DABCO (1.8 equiv.) and with solid (hetero)aryl bromide (1.0 equiv.), and amine (2.0 equiv.), weighed out under air. One cycle of vacuum/argon was performed. The reaction vessel was charged with liquid coupling partners using a Hamilton syringe through a rubber septum, while bubbling argon through the liquid materials. DMA (1 M, corresponding to the heteroaryl bromide) was added using a syringe through a rubber septum, the cap of the vial was switched for a new one, and the reaction tube was placed into a pre-heated oil bath held at 60 °C. The reaction was stirred for 24 h at that temperature and was then allowed to cool down to ambient temperature. The reaction was diluted with EtOAc, quenched by the addition of an aqueous solution of HCl (1 M), followed by neutralizing with a saturated aqueous solution of NaHCO3. A saturated aqueous solution of LiCl was added, and the phases were separated. The organic phase was washed with a saturated aqueous solution of LiCl (3 x), followed by back extraction of the combined aqueous layers with EtOAc (1-2 x). The combined organic layers were dried over MgSO4 and concentrated under reduced pressure. Purification via column chromatography over silica gel afforded the pure desired product.
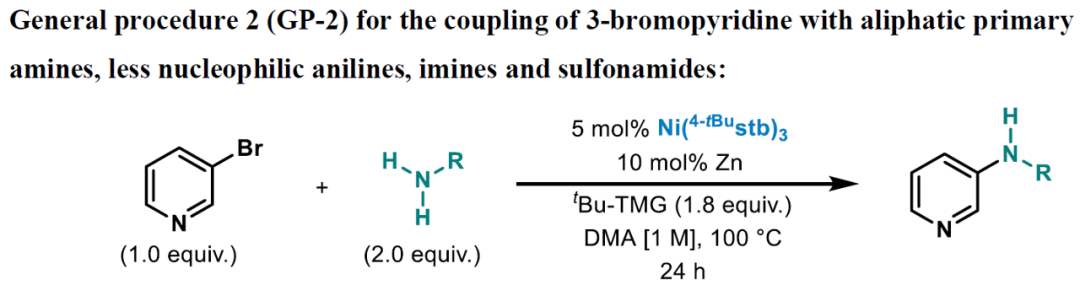
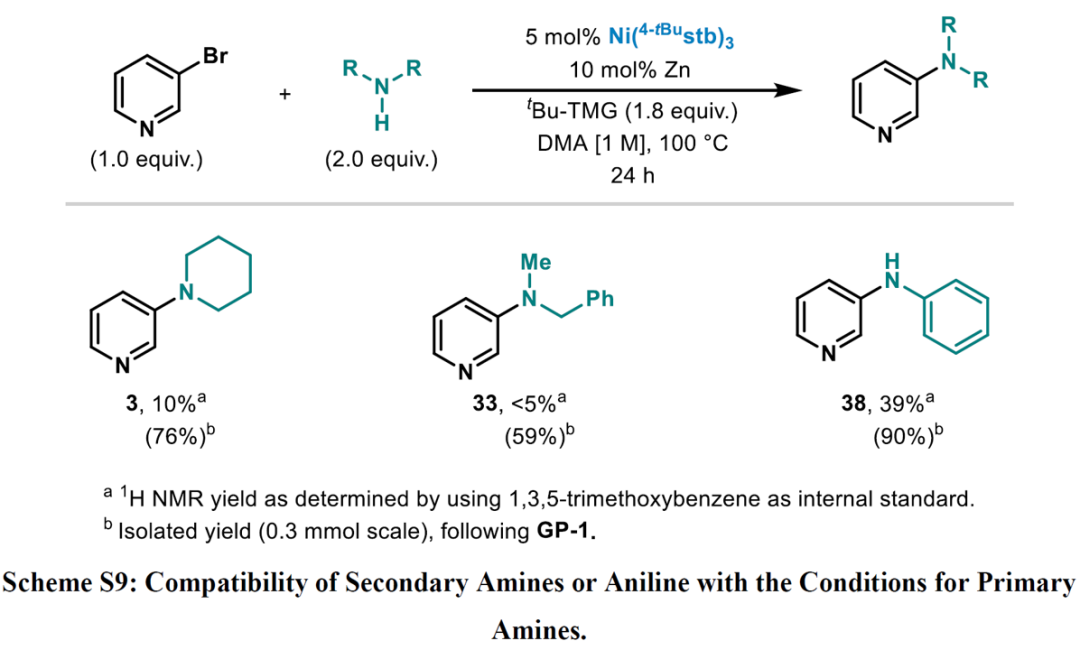
A 12-mL screwcap vial equipped with a stirring bar was dried under vacuum using a heat gun set to 550 °C, followed by three cycles of vacuum/argon. The vial was charged with Ni(4-tBustb)3 (5 mol%), Zn dust (< 60 μm, 10 mol%), and with the solid amine (2.0 equiv.), weighed out under air. One cycle of vacuum/argon was performed. The reaction vessel was charged with 3-bromopyridine (1.0 equiv.) using a Hamilton syringe, tBu-TMG (2-tert-butyl-1,1,3,3-tetramethylguanidine) (1.8 equiv.), and with the liquid amine (2.0 equiv.) through a rubber septum, while bubbling argon through the liquid materials. DMA (1 M, corresponding to 3-bromo pyridine) was added through the rubber septum, the cap of the vial was switched for a new one, and the reaction tube was placed into a pre-heated oil bath held at 100 °C. The reaction was stirred for 24 h at that temperature and was then allowed to cool down to ambient temperature. The reaction was diluted with EtOAc, quenched by the addition of an aqueous solution of HCl (1 M), followed by neutralizing with a saturated aqueous solution of NaHCO3. A saturated aqueous solution of LiCl was added, and the phases were separated. The organic phase was washed with a saturated aqueous solution of LiCl (3 x), followed by back extraction of the combined aqueous layers with EtOAc (1-2 x). The combined organic layers were dried over MgSO4 and concentrated under reduced pressure. Purification via column chromatography over silica gel afforded the pure desired product.
Conclusion
The procedure appears quite complex; it is unclear what the role of zinc powder is; the catalyst (CAS NO.: 2468315-70-8) is available for purchase at a reasonable price, approximately 1000 RMB per gram on a certain platform.
References (the paper is open access, you can click the link at the end to download and read):
Saeb R, Boulenger B, Cornella J. “Naked Nickel”-Catalyzed Amination of Heteroaryl Bromides. Org Lett. 2024; 26(28):5928-5933. doi: 10.1021/acs.orglett.4c01738. PMID: 38967981.