




S. He, Y. Zou, K. Chen, S. P. Jiang. A critical review of key materials and issues in solid oxide cells. Interdiscip Mater. doi:10.1002/idm2.12068
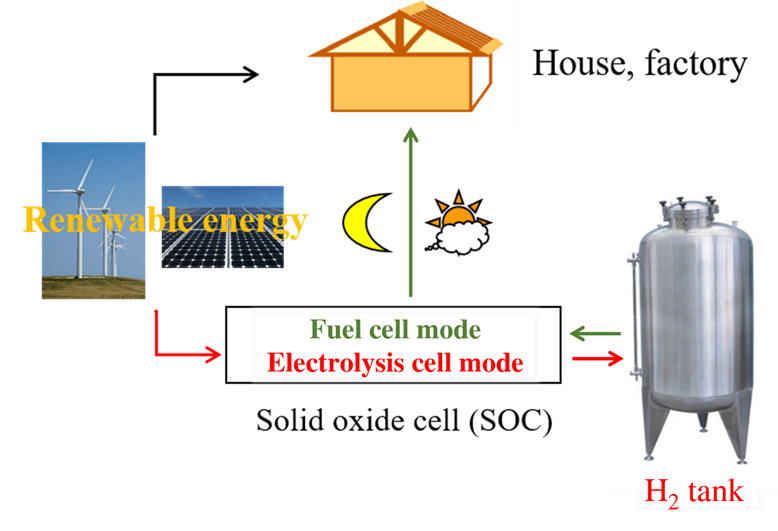
Figure 1. Conceptual diagram of SOC technology that can switch between SOFC and SOEC modes for the storage and regeneration of renewable energy such as solar and wind. The black arrows indicate that households and factories can directly use renewable energy, the red arrows indicate the use of SOEC to store renewable energy in the form of H2, and the green arrows indicate the use of SOFC to generate electricity for households and factories from stored H2.
Due to these unique characteristics, SOC technology is set to become an indispensable part of our future energy system. Therefore, this paper will review the key materials of SOCs (i.e., electrodes, electrolytes, interconnects, and seals) and the latest research progress on critical issues (i.e., including surface chemistry, segregation, electrode/electrolyte interfaces, and different material degradation mechanisms under reversible operation of SOCs). This review will first introduce the basic principles and thermodynamics, then briefly discuss key SOC materials, and finally propose future prospects and challenges for this technology.
1. Working Principles
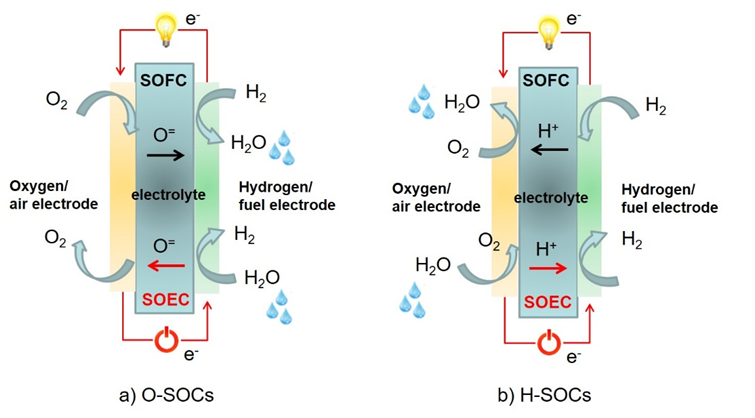
Figure 2. Schematic diagram of solid oxide batteries conducting (a) oxygen ions (O-SOCs) or (b) protons (H-SOCs)
2. Key Materials
2.1 Electrolyte
To meet the requirements of SOC electrolytes, materials need to possess good ionic conductivity (greater than 10-2 Scm-1 under operating conditions) and negligible electronic conductivity. Electrolyte materials typically contribute significantly to the ohmic resistance of the battery, which can significantly affect the overall performance of the battery, especially at low temperatures (e.g., <600°C). Therefore, developing electrolyte materials with high ionic conductivity over a wide temperature range is crucial for upgrading SOC devices.
2.2 Air Electrode
In SOCs, the air electrode catalyzes the oxygen reduction reaction (ORR) in SOFC mode and the oxygen evolution reaction (OER) in SOEC mode, providing pathways for ion migration and mass transfer. Therefore, electrode materials need to exhibit good electrocatalytic activity for ORR/OER, as well as good electronic/ionic conductivity and compatibility with the electrolyte interface.
2.3 Fuel Electrode
In SOCs, the hydrogen electrode catalyzes the hydrogen oxidation reaction (HOR) in SOFC mode and the steam electrolysis in SOEC mode, facilitating the cleavage of H-H or H-O bonds. Nickel oxide is undoubtedly the most commonly used hydrogen electrode material in SOCs due to its excellent catalytic activity, conductivity, and wide availability. As nickel metal does not possess oxide ion conductivity, it is typically mixed with ionic conductors like YSZ, GDC, or BZCY to extend the length of the three-phase reaction interface.
2.4 Interconnect
In SOCs, the interconnect is the component that connects multiple single cells in the stack. For interconnect materials, they are simultaneously exposed to air (or oxygen) and hydrogen (or other hydrocarbon fuels). Therefore, materials used as interconnects need to exhibit excellent tolerance to harsh environments (e.g., highly reducing or oxidizing conditions). Additionally, interconnect materials need to possess excellent conductivity, low ionic conductivity, and a matching compatible thermal expansion coefficient.
2.5 Sealant
Good sealing is crucial in SOCs. Sealant materials are primarily used to isolate fuel and oxidizer. To ensure that fuel and air gases in the electrode chamber do not leak and mix, sealants need to maintain airtightness and stability at high temperatures (600-1000°C) for long-term operation. Depending on the specific operating conditions of SOCs, sealants need to be compatible with the thermal expansion of surrounding components, have high gas tightness, long-term stability in air and reducing environments, chemical compatibility, and electrical insulation.
3. Key Electrode Modification Technologies
3.1 Impregnation Method
The impregnation method is the process of infiltrating functional phases into the air or fuel electrode skeleton. Before impregnation, the electrode skeleton with sufficient porosity needs to be pre-sintered on the electrolyte. The precursor solution of the functional phase is then impregnated into the skeleton using a syringe, with precise control of the loading. After medium-temperature calcination, nano-catalyst particles form and adhere to the skeleton material. By repeating this process, the impregnation load can be increased.
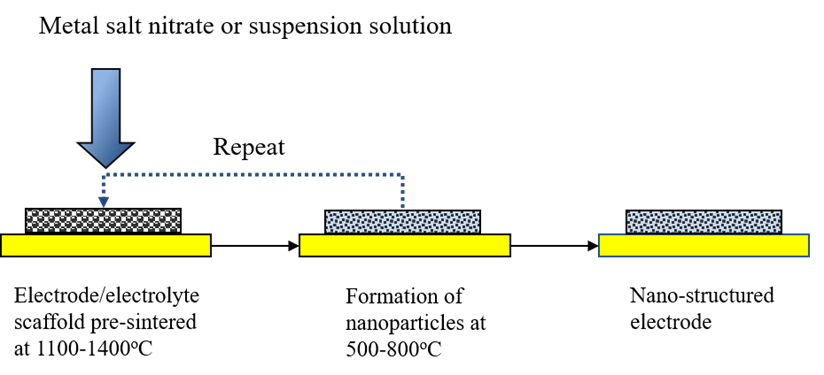
Figure 3. Operational steps of the impregnation method
3.2 In-Situ Leaching Method
In-situ leaching refers to the phenomenon where nanoparticles grow and leach locally from the surface of the skeleton material (usually perovskite) under a reducing atmosphere. The leached nanoparticles are typically reducible transition metals and noble metals (such as Ni, Fe, Co, and Pt), which were doped into the lattice of the skeleton material before leaching. The leached nanoparticles exhibit high catalytic activity and demonstrate excellent resistance to agglomeration and carbon deposition due to their highly embedded interface structure with the skeleton material.
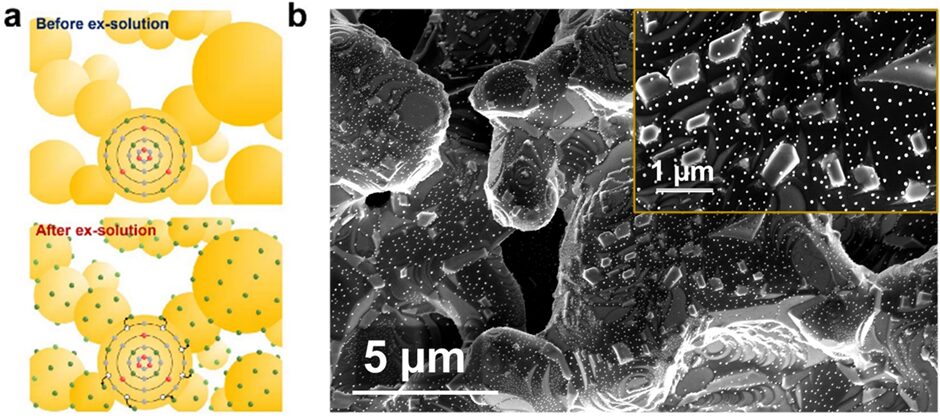
Figure 4. (a) Schematic diagram of structural evolution during the leaching process, (b) Microstructural surface of the material after leaching
3.3 Direct Assembly Method
In 2015, the author’s team developed the direct assembly method for electrodes. This method refers to directly coating the electrode slurry onto the electrolyte without subsequent high-temperature calcination (e.g., >1000°C); subsequently achieving strong electrode/electrolyte interface bonding through in-situ cathodic current polarization at battery operating temperature. The significant advantage of direct assembly technology is that highly active and reactive cobaltate electrodes can be used on YSZ electrolytes without forming harmful resistance phases, as shown in Figure 5. Since the electrode particles are not calcined, they maintain a nanostructure, thus exhibiting good ORR/OER activity.
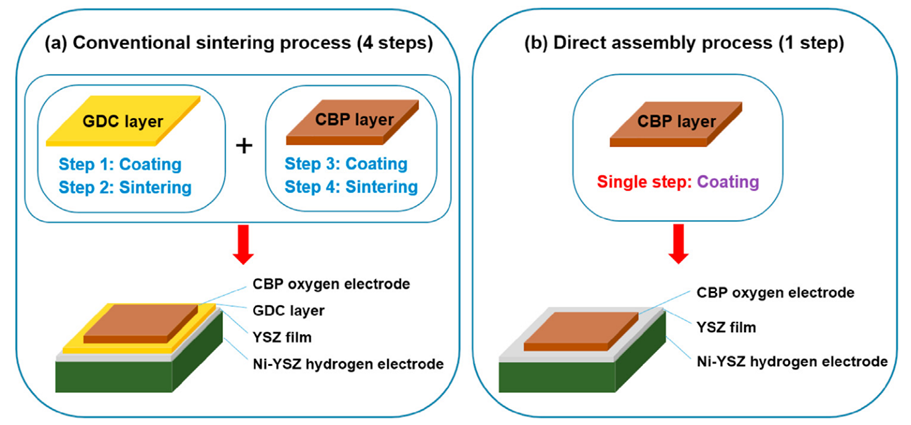
Figure 5. (a) Conventional high-temperature electrode sintering process with GDC barrier layer introduced and (b) flowchart of electrode preparation using direct assembly process
4. Key Issues in SOC
4.1 Microstructural Degradation
A fundamental issue in developing high-performance and long-lifetime SOC devices is the degradation decay of SOC components associated with microstructural changes. Material degradation occurs in SOFC mode, while in SOEC mode, performance degradation typically occurs more rapidly. Unlike the operating conditions of SOFC, under SOEC operating conditions, the electrolyte experiences high oxidative chemical potential near the oxygen electrode side and undergoes harsh reducing atmosphere on the hydrogen electrode side. There exists a significant oxygen partial pressure (PO2) gradient in the electrolyte of SOEC.
4.2 Surface Chemistry, Segregation, Contaminant Deposition, and Poisoning
In SOCs, both reactants and products are in the gas phase, and reactions occur at the electrode and electrolyte surfaces as well as in the electrode/electrolyte interface regions. Therefore, the surface chemistry of electrode and electrolyte materials plays a critical role in the performance and degradation of SOCs. The surface chemistry of solid materials is directly related to surface segregation under open-circuit or polarization conditions. Surface segregation refers to the phenomenon where specific cations redistribute and accumulate on the oxide surface due to the loss of consistency between the surface and the bulk. Accumulation of specific cations on the surface will lead to deviations in the surface stoichiometry/composition from the nominal values of the bulk, resulting in changes in the physicochemical and catalytic properties of the oxide materials. For example, segregated Sr cations on the surface are electrocatalytically inert, thus blocking the active surface for oxygen reduction electrochemical reactions under SOFC operating conditions. Since all electrochemical reactions are related to surface processes, surface segregation is very important for SOCs and plays a key role in the degradation mechanisms of SOC electrodes due to contaminant deposition and poisoning of electrode materials.


Chen Kongfa
Professor at the School of Materials Science and Engineering, Fuzhou University. He obtained his bachelor’s, master’s, and doctoral degrees from Harbin Institute of Technology. Prior to his current position, he served as a postdoctoral researcher at Nanyang Technological University and Curtin University. His research interests lie in solid oxide fuel cells and solid oxide electrolysis cells. He is currently focused on cation surface distribution and the construction of electrode/electrolyte interfaces under the influence of electrochemical polarization.

He Shuai
Assistant researcher at the Advanced Energy Technology Guangdong Laboratory, Xianhu Laboratory, Foshan. He graduated from the School of Materials Science and Engineering, South China University of Technology in 2014 with a bachelor’s degree. In 2015, he pursued his PhD under the supervision of Professor Jiang Sanping in the Department of Chemical Engineering at Curtin University, followed by postdoctoral research in Dr. Yue Xiangling’s research group at the University of St Andrews in the UK. He is dedicated to the development of functional energy materials and solid oxide fuel cells/electrolyzers, as well as the characterization of solid material interfaces based on electron microscopy.

Zou Yuanfeng
Engineer at the Advanced Energy Technology Guangdong Laboratory, Xianhu Laboratory, Foshan, graduated with a master’s degree from Fuzhou University in 2021. His research interests include fuel cells and high-temperature electrolysis.
Interdisciplinary Materials is an open-access high-level academic journal co-founded by Wiley Publishing Group and Wuhan University of Technology. The editor-in-chief is Academician Zhang Qingjie and Academician Fu Zhengyi. The editorial board consists of 30 international distinguished scholars and 43 academicians. Interdisciplinary Materials is the first high-level journal in the field of “interdisciplinary materials” focusing on cutting-edge research at the intersection of materials science and other disciplines such as physics, chemistry, mathematics, mechanics, biology, energy, environment, and information, aiming to publish the latest results in interdisciplinary research involving materials.
· First published in January 2022, with free publication for the first three years
· Included in the DOAJ database in June 2022
· Selected as a “High-Starting New Journal” in the “China Science and Technology Journal Excellence Action Plan” in September 2022
For more information about the journal, please visit:
https://onlinelibrary.wiley.com/journal/2767441X
http://sklwut.whut.edu.cn/im/wz/
https://mc.manuscriptcentral.com/intermat


Long press to recognize the QR code
Learn more about the journal


MaterialsViews
Official WeChat platform of Wiley’s material science journals
Push material research news | Interview material experts and newcomers
Share writing and submission experiences | Follow the latest recruitment information