
PMID: 39891259
DOI: 10.1186/s12974-024-03171-yIF: 11.7 Q1
Abstract
CAAT/Enhancer Binding Protein β (C/EBPβ) is associated with the inflammatory response in neurodegenerative diseases, particularly in the brain. However, the regulatory role of C/EBPβ in spinal cord injury (SCI) and its impact on neurological recovery remain unknown. In this study, the research team observed a significant upregulation of C/EBPβ in microglia following spinal cord injury in mice, which was associated with neuroinflammation. Knockdown of C/EBPβ in the spinal cord attenuated microglial pyroptosis, reduced the production of pro-inflammatory cytokines, and inhibited neuronal apoptosis. Mechanistically, C/EBPβ promoted the transcription of Fcgr1, which is involved in the activation of microglial pyroptosis. In both in vivo and in vitro experiments, knockdown of Cebpb or Fcgr1, or the pyroptosis inhibitor VX765, inhibited neuronal apoptosis in mice and improved neurological recovery. These findings suggest that C/EBPβ acts as a key regulatory factor, participating in microglial pyroptosis-mediated neuroinflammation by activating Fcgr1 transcription. This result was published in the Journal of Neuroinflammation in January 2025.

Research Background
Spinal cord injury (SCI) is a traumatic condition initially induced by direct trauma leading to neuronal damage, but subsequent long-term secondary injury results in persistent and chronic neurological deficits. Effectively alleviating the subsequent secondary neuroinflammatory response is crucial for the repair of SCI. Currently, there is a lack of effective treatment methods for neurological recovery after SCI. Microglia are activated within minutes after SCI, responsible for clearing abnormal cells and debris, and initiating a cascade of inflammatory responses, releasing cytokines and chemokines that can induce neuronal apoptosis and hinder axonal regeneration, thus obstructing neural repair. Pyroptosis, also known as inflammatory necrosis, is a form of programmed cell death induced by inflammatory vesicles, and pyroptosis-induced cellular damage and the ensuing inflammatory response have been identified as key pathogenic factors in various diseases. C/EBPβ is an important member of the C/EBP protein family, and its expression significantly increases in many ischemic or traumatic injury animal models, primarily observed in microglia. Compared to wild-type mice, Cebpb knockout mice exhibited reduced levels of brain infarction and neurological deficits after SCI, indicating that C/EBPβ deficiency has anti-inflammatory and neuroprotective effects.
Currently, other experiments have studied the regulatory role of C/EBPβ in the CNS (especially the brain), but its localization and expression patterns, as well as how it participates in regulation during SCI, remain unclear. Therefore, this study aims to explore the role of C/EBPβ in microglial pyroptosis after SCI and assess whether C/EBPβ deficiency can enhance neurological repair after SCI.

Research Results
C/EBPβ is upregulated in microglia after SCI
The team first used bioinformatics methods to analyze the GEO database and found that the GSE92657 dataset contains expression data of Cebpb before and after SCI in mice. Volcano plots revealed a large number of DEGs between the SCI group and the SHAM group (Figure A). It also showed that Cebpb was significantly upregulated in the SCI group compared to the SHAM group (Figure B). Meanwhile, KEGG enrichment analysis indicated that the high expression group of Cebpb was mainly associated with inflammatory cytokine-induced signaling pathways (Figure C). Next, SCI modeling was completed in mice, and expression was detected multiple times. RT-qPCR and western blotting showed that Cebpb expression gradually increased from day 1 to day 7 after SCI, peaking around day 7 and then gradually decreasing (Figures E, F). Immunofluorescence staining of spinal cord tissue on day 7 post-injury showed significant infiltration of microglia and astrocytes in the injured area (Figure G). Notably, C/EBPβ was most prominently expressed in infiltrating microglia, followed by astrocytes, and was rarely detected in neuronal cells.

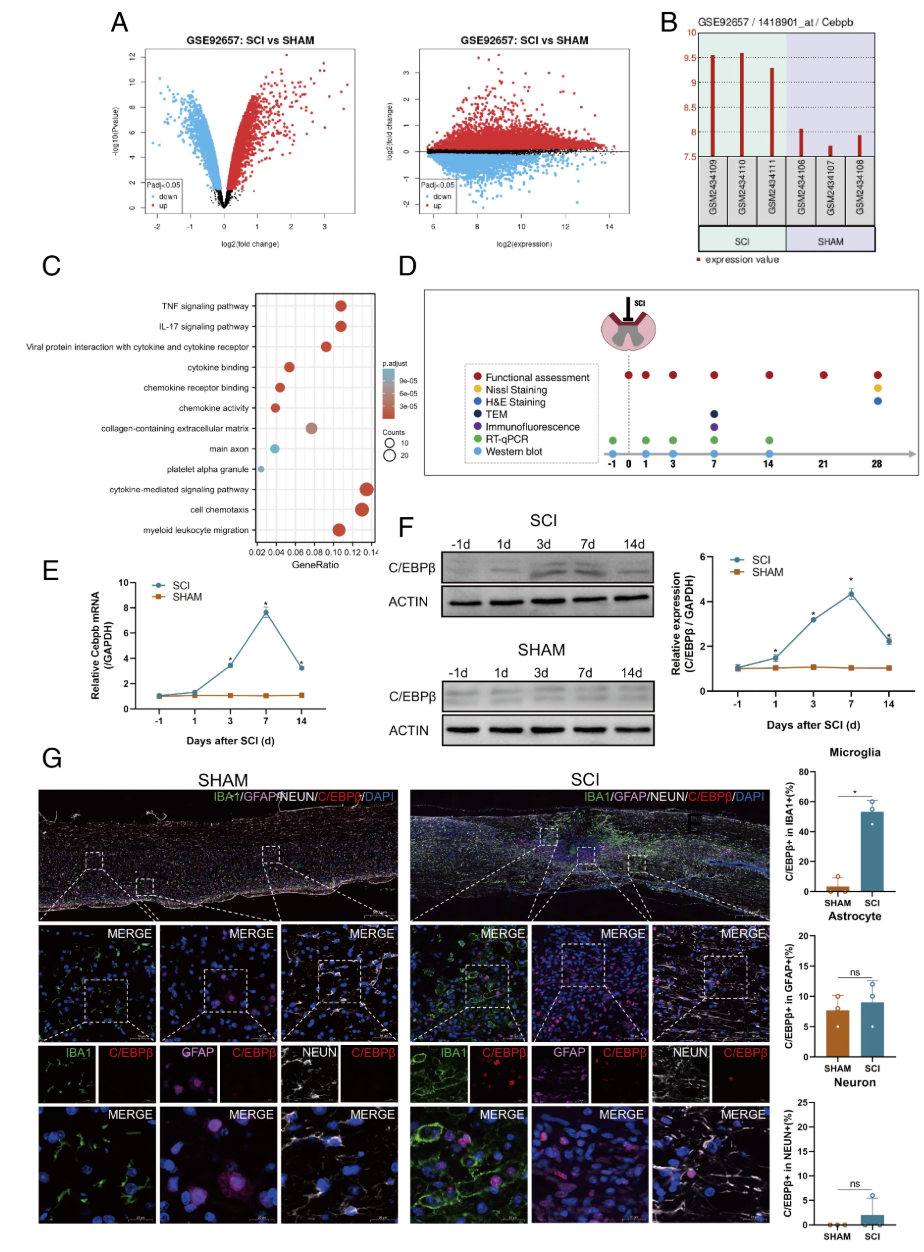
Figure1
C/EBPβ activates microglial pyroptosis in vitro and in vivo Next, the activation of pyroptosis in the injured spinal cord was assessed. Western blot analysis showed significant upregulation of NLRP3 and GSDMD-N after SCI, and RT-qPCR results were consistent with western blot results. Immunofluorescence staining of IBA1 and GSDMD-N showed a significant increase in double-positive cells in the SCI group, indicating microglial pyroptosis after SCI. Additionally, gene knockdown was achieved using RNA interference, with intramedullary injection of shCebpb to reduce its expression. Results from western blot, RT-qPCR, and immunofluorescence assays showed a decrease in apoptosis marker levels in the shCebpb group. In vitro experiments showed that pyroptosis markers were significantly upregulated in LPS-activated BV2 cells, and the secretion of pyroptosis-related inflammatory factors IL-18, IL-1β, IL-6, and TNF-α was significantly increased. The levels of these pyroptosis markers and inflammatory factors in the shCebpb group were significantly lower than those in the SCI group. These findings indicate that C/EBPβ upregulation activates microglial apoptosis after SCI.

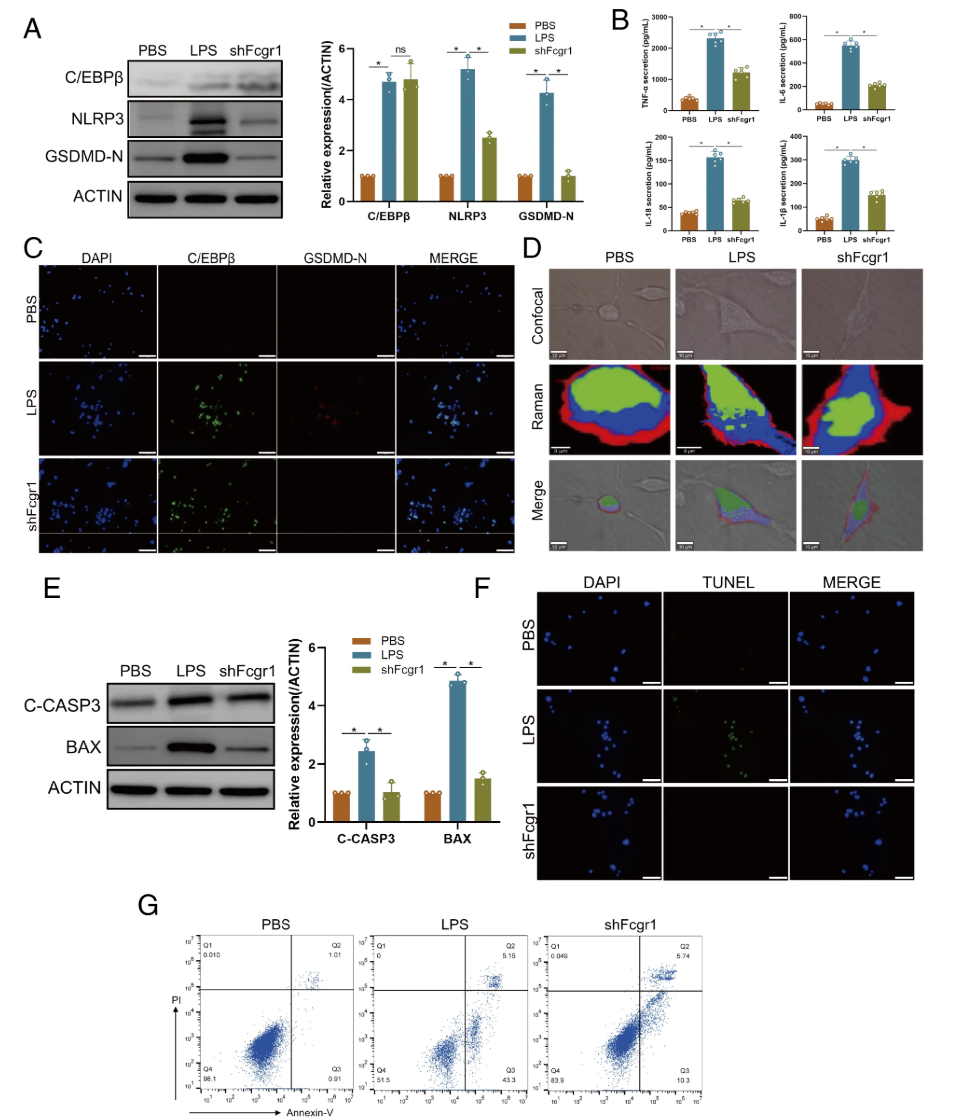
Figure2
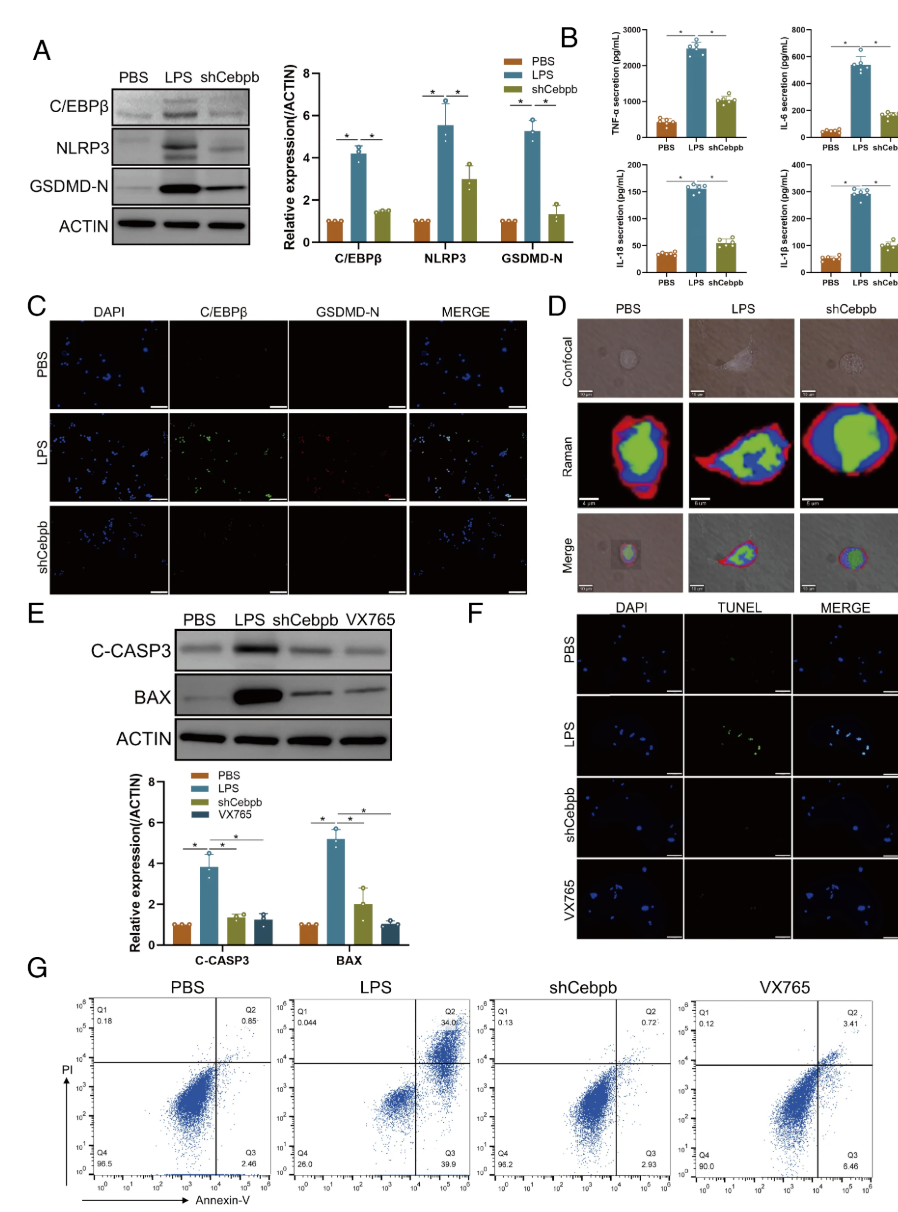
Figure3
C/EBPβ-induced microglial pyroptosis leads to neuronal apoptosis
The association between the reduction of neurological function after SCI and C/EBPβ-mediated microglial pyroptosis was studied by intervening with the pyroptosis inhibitor VX765. Experiments were conducted on SCI mice, and the BMS score and rotarod test were used to assess the recovery of motor function in each group of mice after SCI. Mice in the SCI group showed the poorest recovery, while mice in the SHCEBPB and VX765 groups showed significant recovery starting from day 14 (Figures A and B). Additionally, footprint analysis on day 28 showed that the hind limb movement was most affected in the SCI group, while the shCebpb and VX765 groups exhibited significant recovery (Figure C). Immunofluorescence staining indicated that the level of neuronal C-CASP3 was most pronounced in the SCI group compared to other groups (Figure D). Western blotting results showed that both the shCebpb and VX765 groups exhibited significant inhibition of the upregulation of apoptosis-related proteins observed in the SCI group (Figure E). Nissl staining results indicated that the number of Nissl bodies in the shCebpb and VX765 groups was significantly higher than that in the SCI group (Figure F). Collectively, these results suggest that C/EBPβ-mediated microglial pyroptosis induces neuronal apoptosis. In contrast, the absence of C/EBPβ or inhibition of pyroptosis significantly promotes recovery of motor function after SCI.

Figure4
C/EBPβ regulates the expression of Fcgr1 in microglia as a transcriptional activator
Using “Cebpb” and “BV2 cells” as target genes and target cells to probe the Cistrome DB database, four genes with the highest C/EBPβ binding scores were identified, all belonging to the Fcgr family. Flow cytometry analysis indicated that the level of FCGR1 (CD64) in the LPS group was significantly higher than that in the PBS group, while the levels of FCGR2 and FCGR3 (CD16/32) showed no difference (Figure C). Using the Jaspar website, two potential C/EBPβ binding sites in the promoter region of FCGR1 were predicted (Figure D). Western blotting showed that FCGR1 was downregulated when SHCEBPB was knocked down in BV2 cells (Figure E). To investigate whether C/EBPβ transcriptionally regulates FCGR1, ChIP (chromatin immunoprecipitation) and dual-luciferase reporter assays were performed. The results indicated that C/EBPβ was significantly enriched in the promoter region of FCGR1 (Figure F). Gene assays showed that C/EBPβ binds to the P2 site of the FCGR1 promoter, thereby promoting FCGR1 transcription. These findings collectively indicate that C/EBPβ acts as a transcriptional activator, upregulating the expression of Fcgr1.


Figure5
C/EBPβ activates microglial pyroptosis by activating Fcgr1 transcription Finally, in vivo and in vitro experiments were conducted to test the findings regarding C/EBPβ-induced Fcgr1 transcription activation and microglial pyroptosis. It was found that transfection of shFcgr1 into BV2 cells effectively inhibited the upregulation of pyroptosis-related markers and the secretion of pro-inflammatory factors induced by LPS. Results from western blot analysis, TUNEL staining, and flow cytometry assays collectively indicated that apoptosis in N2a cells in the shFcgr1 group was reversed. These findings collectively suggest that Fcgr1 is involved in C/EBPβ-induced microglial pyroptosis. In in vivo tests, the shFcgr1 group showed significantly lower expression levels of pyroptosis markers and inflammatory factors at both protein and mRNA levels compared to the negative control group, and motor function assessments indicated that the shFcgr1 group of mice showed significant recovery of motor function by day 21 post-SCI. Multiple results collectively indicate that C/EBPβ activates microglial pyroptosis by activating Fcgr1 transcription.


Figure6
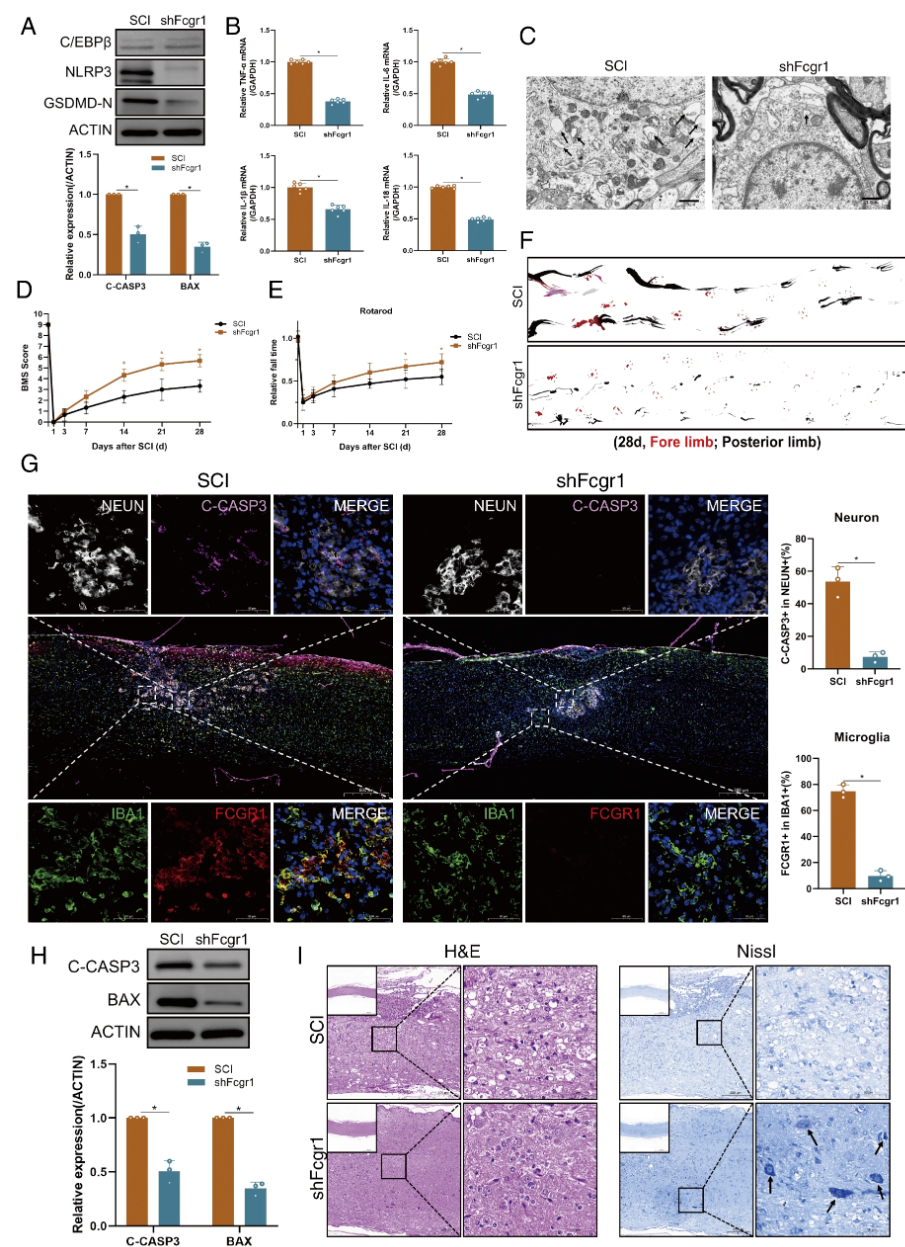
Figure7
Conclusion
In this study, the author team observed a significant upregulation of C/EBPβ in microglia in a SCI mouse model. The results indicate that the C/EBPβ-Fcgr1 axis participates in the neuroinflammatory response after SCI by activating microglial pyroptosis. Administration of the pyroptosis inhibitor VX765 or knockdown of Cebpb or Fcgr1 expression in the spinal cord effectively inhibited microglial pyroptosis, suppressed the secretion of pro-inflammatory cytokines and neuronal apoptosis, and promoted neural repair after SCI. In summary, the findings suggest that inhibiting the C/EBPβ-Fcgr1 axis could be a therapeutic strategy to alleviate microglial neuroinflammatory responses and promote neural repair after SCI.
 The source of this article is from the internet. If there is any infringement, please contact us for removal!
The source of this article is from the internet. If there is any infringement, please contact us for removal!