
Introduction
Zongertinib (BI1810631) is a selective HER2 (ERBB2) tyrosine kinase inhibitor (TKI) developed by Boehringer Ingelheim, which covalently binds to the receptor tyrosine kinase domain (TKD) of HER2 exon 20 mutations. This selectivity allows it to block only the abnormal downstream signaling of HER2 without affecting the wild-type epidermal growth factor receptor (wtEGFR, i.e., normal EGFR) signaling pathway, thereby avoiding the common dose-limiting toxicities associated with other pan-HER2 inhibitors that target wild-type EGFR.
On January 15, 2025, the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) of China announced that the application for marketing authorization of zongertinib has been accepted. The drug has recently been officially included in priority review by the CDE, with the indication for the treatment of adult patients with unresectable or metastatic non-small cell lung cancer (NSCLC) carrying HER2 activating mutations who have previously received systemic therapy. This indication approval is based on the results of the Beamion LUNG-1 study.
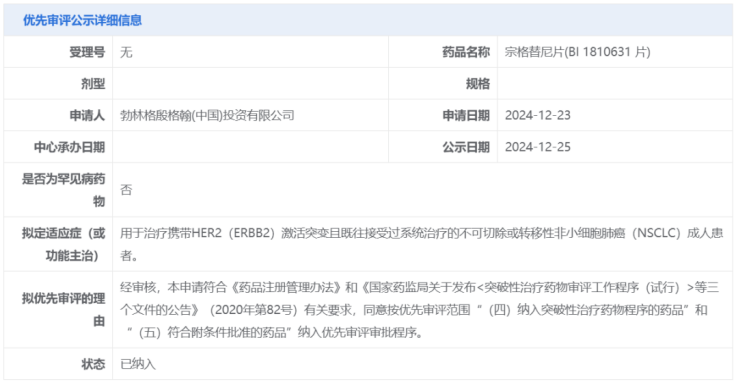
Figure 1. Detailed information on the priority review of zongertinib (from the CDE official website)
Recently, the 2025 American Association for Cancer Research (AACR) published the results of three cohorts from this study, which were simultaneously published online in the New England Journal of Medicine (NEJM).
Need for More Anti-HER2 Treatment Options for HER2 Positive Lung Cancer
In NSCLC, the mutation rate of HER2 is 2% to 4%, with TKD mutations being the most common, particularly insertions in exon 20. Other mutations exhibit high heterogeneity, primarily distributed in the extracellular and transmembrane domains of the receptor.
Currently, the only approved anti-HER2 targeted drug in the treatment landscape for HER2 positive NSCLC globally (by regulatory bodies such as the NMPA and FDA) is antibody-drug conjugates (ADC). Targeting HER2 ADC has become the standard treatment due to its sustained anti-tumor activity; however, there is an urgent need for more diverse treatment options for HER2 positive NSCLC patients.
Although pan-HER TKIs have shown significant efficacy in cancers such as breast cancer, their performance in HER2 mutant NSCLC has been unsatisfactory and often accompanied by EGFR-related toxicities (such as diarrhea and rash).
The novel drug Zongertinib (BI 1810631), as an oral irreversible TKI, selectively inhibits HER2 receptor kinase activity while avoiding interference with EGFR receptor kinase, effectively circumventing the toxic drawbacks of traditional pan-HER2 TKIs. The ongoing Phase Ia-Ib Beamion LUNG-1 study aims to evaluate the safety and efficacy of this drug in advanced solid tumors with HER2 mutations. The dose escalation data from Phase Ia shows that at the recommended expansion doses (120 mg and 240 mg once daily), the drug’s toxicity profile is excellent, with a low incidence of grade 3 or higher toxic events and initial anti-tumor activity.
This report focuses on the data from three cohorts in the Phase Ib dose expansion stage, further analyzing the efficacy and safety of zongertinib in previously treated patients with HER2 mutant advanced/metastatic NSCLC, aiming to facilitate discussion and learning among readers!
Beamion LUNG-1 Study Results: Focus on Advanced/Metastatic HER2 Positive NSCLC
Beamion LUNG-1 is a Phase Ia–Ib clinical trial, and this report presents data from the Phase Ib stage concerning three cohorts of patients with treated HER2 mutant advanced/metastatic NSCLC:
-
Cohort 1: TKD mutation non-squamous carcinoma;
-
Cohort 5: HER2 ADC treated TKD mutation non-squamous carcinoma;
-
Exploratory Cohort 3: Non-TKD mutation non-squamous carcinoma or TKD mutation squamous carcinoma.
The study enrollment criteria included patients aged ≥18 years, histologically confirmed HER2 mutations, measurable lesions per RECIST 1.1, ECOG 0-1, and having received ≥1 line of systemic therapy (including platinum-based chemotherapy). Patients with stable brain metastases were allowed to enroll, but mutations must be confirmed by Oncomine Dx Target Test.
In the initial phase, researchers randomly assigned patients in Cohort 1 to receive Zongertinib treatment at 120 mg or 240 mg once daily in a 1:1 ratio; Cohorts 3 and 5 received Zongertinib at 240 mg once daily. After the dose selection analysis in Cohort 1, all cohorts uniformly adopted the selected dose (120 mg/d), with treatment continuing until disease progression or intolerable toxicity.
The primary endpoint of the study was the objective response rate (ORR) assessed by blinded independent central review (Cohorts 1 or 5) or investigator review (Cohort 3) per RECIST 1.1, with secondary endpoints including duration of response, progression-free survival, and intracranial response in patients with brain metastases (RANO-BM criteria), with safety events assessed according to CTCAE 5.0.
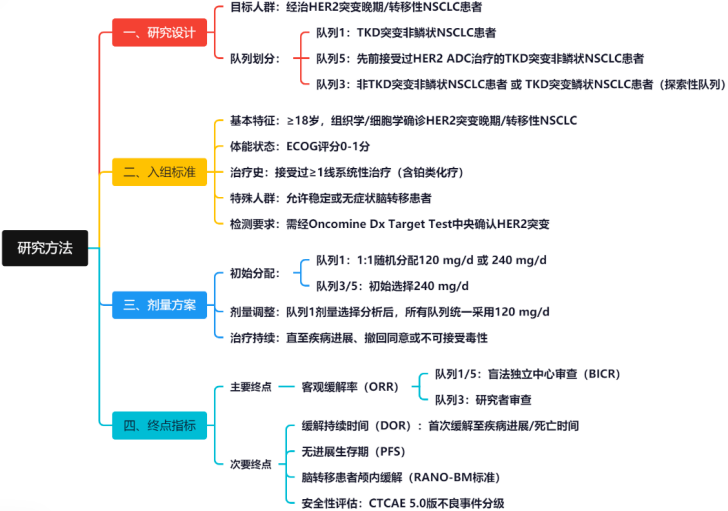 Figure 2. Summary of Study Methods
Figure 2. Summary of Study Methods
In terms of statistical methods, during the interim dose selection analysis phase, it was anticipated that there would be 20 patients in each of the 120 mg and 240 mg dose groups of Cohort 1 who would complete one baseline tumor assessment or terminate treatment. Throughout the trial, it was expected that 60 patients would be enrolled in each of the two dose groups of Cohort 1 to ensure sufficient power to reject the null hypothesis of an ORR ≤30%. Cohort 5 was expected to enroll 30 patients to ensure sufficient power to reject the null hypothesis of an ORR ≤25%. Exploratory Cohort 3 was expected to enroll 30 patients to ensure sufficient power for efficacy signal detection (i.e., ORR ≥40%). Time-to-event endpoints were analyzed using the Kaplan-Meier method, and safety data were subjected to descriptive statistics.
Study Results
From March 8, 2023, to November 29, 2024, a total of 132 patients participated in the study, including 75 in Cohort 1 (TKD mutation), 31 in Cohort 5 (previously treated with HER2 targeted therapy TKD mutation), and 20 in Cohort 3 (non-TKD mutation). After the dose selection analysis in Cohort 1, the final selected dose was 120 mg, as this dose exhibited better safety with similar efficacy.
-
Patients untreated with ADC achieved an ORR of up to 71% with zongertinib
In Cohort 1 (untreated with ADC and TKD mutation), 75 patients received 120 mg zongertinib treatment, with a centrally confirmed ORR of 71% (95% CI: 60%-80%; rejecting the null hypothesis, P<0.0001), a median duration of response (DoR) of 14.1 months, and a median progression-free survival (PFS) of 12.4 months. Subgroup analysis showed that in patients with baseline brain metastases (37%), the ORR was 64%, with an intracranial ORR of 41%. The ORR in common mutation subgroups (e.g., A775_G776insYVMA) reached 81%. The efficacy data for the 240 mg dose group was comparable to that of the 120 mg dose group, with an ORR of 84% (95% CI: 72%-91%), median DoR and median PFS of 9.7 months and 10.9 months, respectively, and an intracranial ORR of 42% in patients with brain metastases.
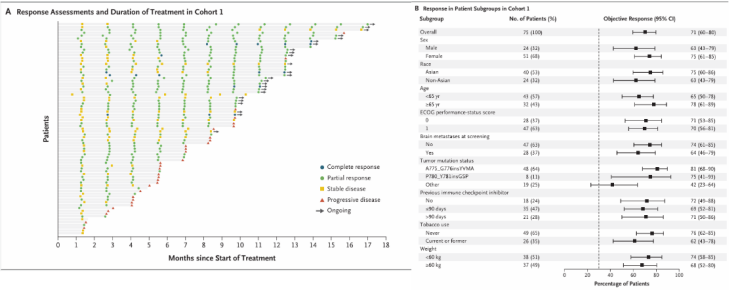
Figure 3. ORR and Subgroup Analysis of Patients in Cohort 1
-
Patients previously treated with trastuzumab deruxtecan achieved an ORR of 41% with zongertinib
Cohort 5 (ADC treated and TKD mutation) enrolled 31 patients, with an ORR of 48% (95% CI: 32%-65%), median DoR and median PFS of 5.3 months (95% CI: 2.8-NE) and 6.8 months (95% CI: 25.4-NE), respectively. Among the 22 patients previously treated with trastuzumab deruxtecan, the ORR was 41% (95% CI: 23%-61%).
In exploratory Cohort 3 with 20 non-TKD mutation patients, the ORR was 30% (95% CI: 15%-52%), with median DoR and PFS still immature.
-
Zongertinib demonstrates good safety profile
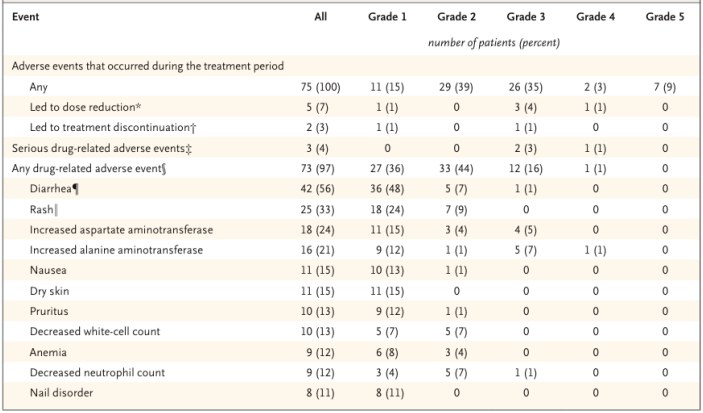
In terms of safety, patients exhibited good tolerance to zongertinib. Among the 75 patients in Cohort 1 receiving 120 mg zongertinib treatment, 97% reported drug-related adverse events, but no drug-related interstitial lung disease or toxic effects associated with interstitial lung disease were reported. Thirteen patients (17%) reported grade ≥3 drug-related adverse events (TRAE), with the most common grade ≥3 TRAEs being elevated alanine aminotransferase levels (8%) and elevated aspartate aminotransferase levels (5%). Seven patients (9%) reported serious adverse events, but none were considered related to zongertinib. Overall, the incidence of TRAEs such as diarrhea (56%) and rash (33%) was high, but most were mild grade 1-2, with only one patient requiring a dose reduction due to diarrhea.
Table 1. Summary of Study Safety Results
 Conclusion:
Conclusion:
In this Phase Ib clinical trial, zongertinib demonstrated sustained clinical benefits in previously treated patients with HER2 mutant non-squamous NSCLC, with good tolerability.
Compared to the previously approved dose of 5.4 mg/kg trastuzumab deruxtecan in the DESTINY-Lung02 study, zongertinib showed an improvement in ORR (71% vs. 49%), extended mPFS (12.4 months vs. 9.9 months); in terms of safety, the incidence of grade ≥3 TRAEs was lower (17% vs. 39%), and no risk of interstitial pneumonia was observed. Furthermore, in patients who progressed after trastuzumab deruxtecan treatment, zongertinib still achieved an ORR of 41%, suggesting its potential to overcome certain ADC resistance mechanisms.
However, it is worth noting that as an open-label, uncontrolled Phase 1 trial, the level of evidence from this study is limited. The ongoing Phase 3 Beamion LUNG-2 study (NCT06151574) will further validate the efficacy and safety of zongertinib in first-line treatment of HER2 mutant NSCLC.
References[1] Beamion LUNG-1 Investigators. Zongertinib in Previously Treated HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med. 2025 Apr 28. doi: 10.1056/NEJMoa2503704. Epub ahead of print.[2] Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non–small-cell lung cancer. N Engl J Med 2022;386:241-251.Compiled by: Mao Yang; Edited by: BreeCover Image: Tuchong CreativeSubmission: [email protected]