
Antibody-drug conjugates (ADCs) consist of antibodies, linkers, and cytotoxic payloads. Compared to traditional chemotherapy, ADCs target tumors with the promise of reducing systemic toxicity, providing a broader therapeutic window and a higher therapeutic index. Over the past decade, ADCs have gradually matured in the treatment of solid tumors and hematological malignancies, becoming a revolutionary field in anti-tumor drug research.
Although the development of ADCs has achieved significant success, the emergence of unexpected toxicity and resistance poses enormous challenges. Various factors have led to widespread clinical dilemmas, especially in solid tumors. These factors include: (1) the heterogeneity of solid tumors limits the clinical efficacy of targeting a single antigen; (2) treatment pressure induces antigen downregulation, epitope mutations, and the activation of bypass pathways, leading to significant ADC resistance; (3) target antigen expression in normal tissues, linker instability, and other variables lead to off-target toxicity; (4) resistance to internalization of target antigens hinders sufficient therapeutic effect. Therefore, the optimization of antibodies, linkers, and payloads has become a challenge for the next generation of ADCs.
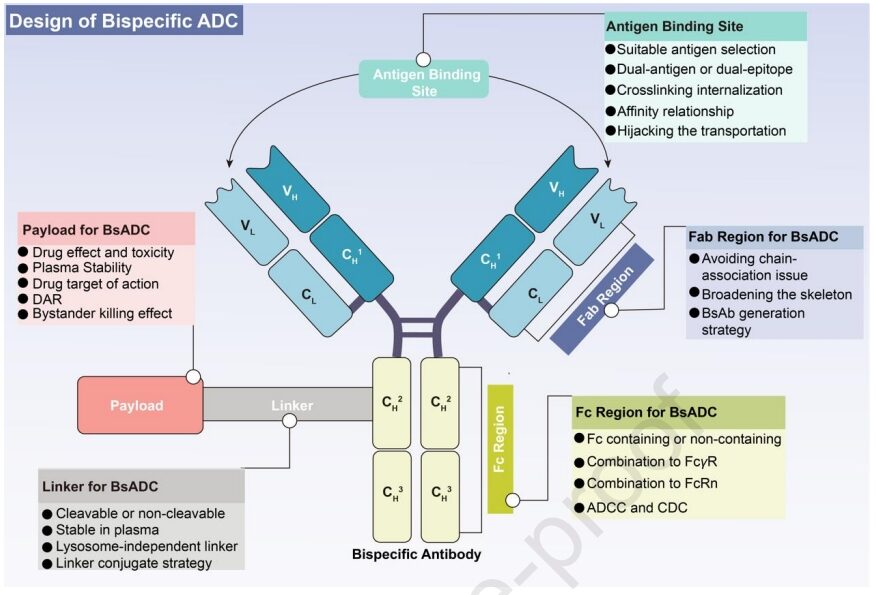
A prospective approach to addressing the above clinical challenges is to couple bispecific antibodies (BsAbs) with linker-payload complexes, resulting in bispecific antibody-drug conjugates (BsADCs). Compared to traditional ADCs, the unique dual epitope/target binding mode of BsADCs not only allows binding to co-expressed antigens in solid tumors to enhance selectivity but also significantly improves internalization. These unique advantages position BsADCs as an important force in the next generation of ADCs. Currently, at least 10 types of BsADCs are undergoing clinical trials. The design of BsADCs is not merely “1+1=2”; the change in binding mode affects overall efficacy, including the comprehensive coordination and optimization of BsAbs, linkers, and payloads.
Currently, the known targets of BsADCs are primarily focused on HER2, EGFR, and c-MET. Each component of the ADC, including the antibody, linker, and payload, requires independent optimization, and any slight modification to any of these key components can lead to substantial changes in clinical characteristics. Therefore, when designing future BsADCs, the optimization and coupling strategy of the antibody-linker-payload complex should be viewed as an interconnected network, requiring a holistic approach.
Bispecific Antibodies
The primary consideration in constructing BsADCs is the wise selection of appropriate target combinations. Target selection is a fundamental prerequisite for the successful development of ADCs and has a critical impact on the final therapeutic window and systemic toxicity. Given the common off-target toxicity and clinical resistance faced by traditional ADCs, the following criteria can help guide target selection:
(1) Traditional target selection focuses on good internalization characteristics, relatively low expression in normal tissues, and high expression in tumors. (2) Given the heterogeneity of solid tumors, it is essential to determine the expression levels of targets in various tumor subtypes and sites to facilitate the optimal implementation of tailored drug delivery, rather than relying on a single “magic bullet.” (3) Due to the unique dual-targeting properties of BsADCs, it is crucial to comprehensively consider the deep effects of antigen combinations. This includes factors such as internalization, recycling, turnover rate, lysosomal degradation, and inherent mechanisms. The integration of these factors is essential for the effective design of BsADCs.
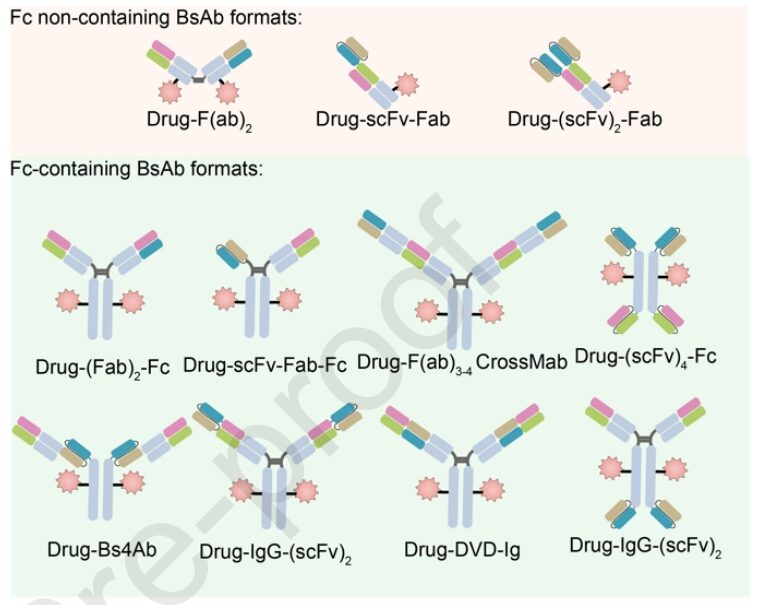
Moreover, one of the main classification criteria for BsADCs is whether they contain an Fc region. Designs without Fc face challenges such as low stability, aggregation issues, and lack of coupling sites. On the other hand, BsADCs containing Fc provide additional advantages such as ADCC, CDC, immune phagocytosis, and cytokine release, all of which contribute to tumor killing. In summary, strategies for constructing Fc regions include: (1) Fc engineering modifications, such as amino acid mutations and glycosylation modifications in the Fc region, which can help mitigate off-target toxicity caused by FcγR binding; (2) Retaining ADCC and CDC: the dual-target binding mode favors the formation of hexamers, which can enhance ADCC and CDC effects, improving tumor killing; (3) Retaining FcRn binding or applying antibody engineering helps increase half-life and safety.
Linkers
The linker in BsADCs is the crucial connection between the antibody and the cytotoxic payload, playing a vital role in payload release and drug stability. An ideal linker should exhibit stability in plasma while promoting effective release in tumors. Currently, the linkers in ADCs can be categorized into cleavable and non-cleavable linkers.
Non-cleavable linkers have high stability in plasma and can only degrade in lysosomes to release the payload. This type of linker results in lower off-target toxicity, increased plasma half-life, and enhanced safety. However, potential resistance may arise from obstacles in internalization and lysosomal transport. BsADCs based on non-cleavable linkers should focus on further optimizing internalization, as well as subsequent endosomal transport and lysosomal degradation.
Compared to non-cleavable linkers, ADCs based on cleavable linkers have a broader range of applications. The main challenge of cleavable linkers lies in off-target toxicity caused by non-specific release. To design lysosome-independent BsADCs, certain cleavable linkers (e.g., Val-Cit linker) can effectively facilitate payload release in early and late endosomes. Moreover, recent proposals for non-internalizing ADCs, where chemical or enzymatic cleavage is triggered extracellularly, can target antigens in the TME and vascular system, activating bystander killing effects.
Payloads
Cytotoxic payloads largely determine overall anti-tumor efficacy and potential adverse reactions. Considering the low permeability of ADCs, the ideal payload for ADCs needs to demonstrate high potency at nanomolar to picomolar levels. Furthermore, these payloads should exhibit sufficient plasma stability, low immunogenicity, and appropriate solubility. Finally, payloads should possess available groups for coupling with antibodies.
The bystander killing effect of payloads in BsADCs is a key aspect worthy of discussion. The bystander killing effect refers to the ability of the payload to kill adjacent non-target cells after release. This is a double-edged sword for the PK/PD characteristics of ADCs. While the bystander killing effect can enhance the overall efficacy of ADCs in heterogeneous tumor environments, it also poses the risk of off-target killing in normal tissues surrounding the tumor. This effect relies on cleavable linkers and hydrophobic payloads. If one of the target antigens is expressed at a certain level in normal tissues (such as c-Met), BsADCs should avoid applying payloads with bystander effects. In summary, the bystander killing effect of BsADCs is expected to overcome tumor heterogeneity, tumor barriers, and poor internalization. However, potential safety issues related to off-target effects need to be carefully considered.
Furthermore, novel drug payloads are crucial for the development of BsADCs. Emerging payloads, such as PROTAC, ferroptosis inducers, oligonucleotides, etc., contribute to the expansion of drug options in the BsADC field. The development of new drugs can significantly enrich the selective drug types in the BsADCs field.
ZW49
ZW49 is based on Zanidatamab, utilizing inter-chain disulfide bond cysteine and protease-cleavable linkers, conjugated with N-acyl sulfonamide auristatin, resulting in good tolerance. The bispecific antibody nature of ZW49 aids in better internalization, and its Fc region confers ADCC, ADCP, and CDC effects. This design addresses several unmet clinical needs in patients expressing HER2.
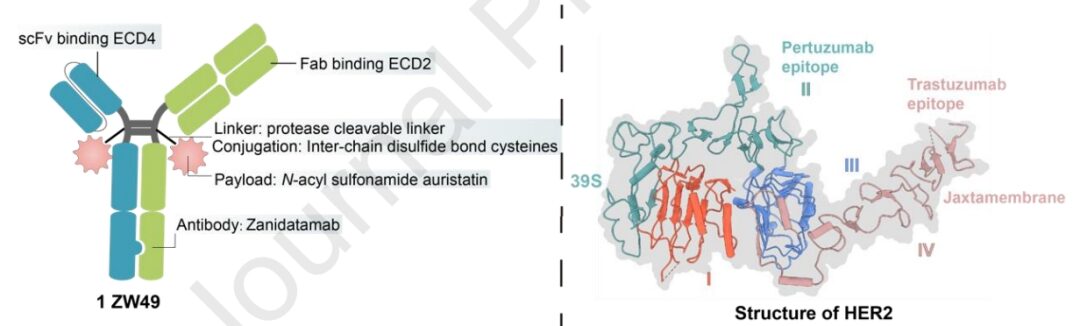
Preclinical data indicate that ZW49 exhibits strong tumor-killing effects and good tolerance without compromising HER2 affinity. Currently, ZW49 is undergoing phase I clinical trials. As of September 2022, disclosed clinical trial data show an objective response rate (ORR) of 31% in patients with advanced solid tumors expressing HER2, while its ocular toxicity profile is notable (keratitis at 42%).
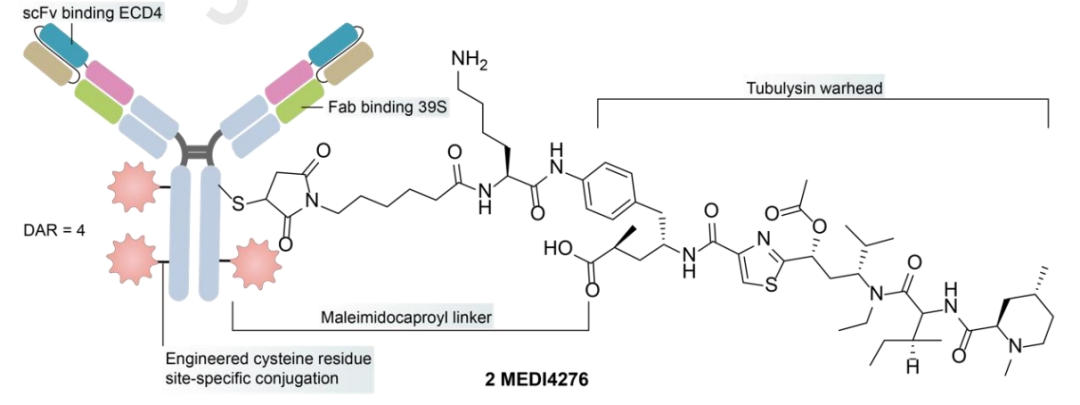
MEDI4276
MEDI4276 is a tetravalent HER2-targeting ADC that fuses the scFv of trastuzumab with the N-terminus of another anti-HER2 IgG1 antibody 39S. MEDI4276 showed significant activity in mouse xenograft models of refractory HER2+ cancers, but did not demonstrate a good efficacy-safety balance in clinical testing – overall ORR in breast cancer patients was low (9.4%), with a maximum tolerated dose (MTD) determined at 0.75 mg/kg every three weeks. Compared to the safety of ZW49, the lower MTD of MEDI4276 may be influenced by its valency, payload, and antibody configuration, indicating the need for further optimization.
Targeting HER2◊CD63 BsADCs
CD63 is a member of the tetraspanin superfamily, exhibiting widespread but not ubiquitous expression. It is primarily localized on the cell surface, late endosomes, and lysosomes. The presence of CD63 in these cellular compartments makes it a potential target for BsADCs, aimed at enhancing internalization and lysosomal transport, ultimately improving drug delivery and therapeutic effects.
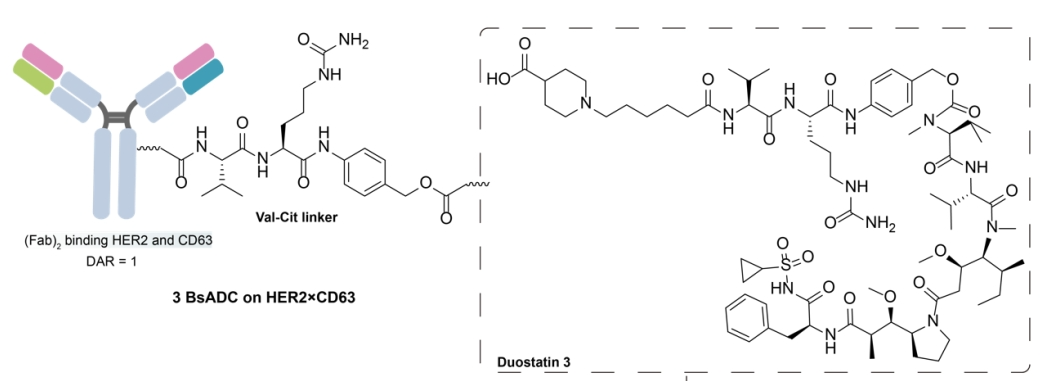
By combining a low-affinity mutant arm of CD63 with another Fab arm from HER2 antibodies, a HER2×CD63 BsAb was generated. This design leverages antibody-dependent receptor crosslinking to enhance effective internalization of HER2 and promote lysosomal co-localization. Subsequently, the Her2×CD63 BsAb was conjugated with the mitotic payload duostatin-3 via a VC linker. However, the efficacy of this BsADC observed in low HER2 tumors was insufficient, indicating the need for further optimization, including potential enhancement of DAR and addressing tumor heterogeneity.
Targeting HER2◊PRLR BsADCs
The prolactin receptor (PRLR) is an overexpressed target in malignant mammary epithelium, effectively mediating clathrin-dependent initial internalization and lysosomal transport through self-ubiquitination and stimulation of AP2 complex recruitment.
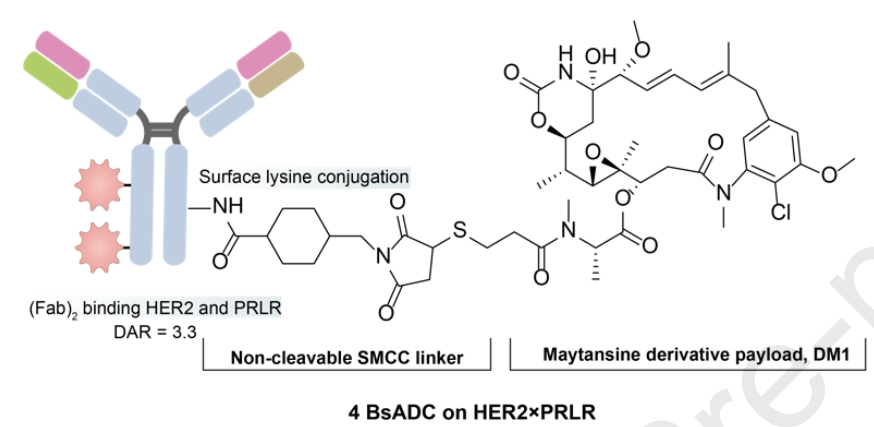
By utilizing the “KIH” method, a BsAb with HER2 and PRLR arms was designed. The BsADC was conjugated with the non-cleavable linker (SMCC) to DM1 via surface lysines, with an average DAR of 3.324. Compared to the high-expressing HER2, the lower expression of PRLR on the cell surface is sufficient to mediate constitutive internalization and subsequent lysosomal degradation. This indicates that even at low expression, high-turnover surface targets can mediate good internalization and lysosomal degradation to enhance the efficacy of BsADCs.
Targeting HER2◊APLP2 BsADCs
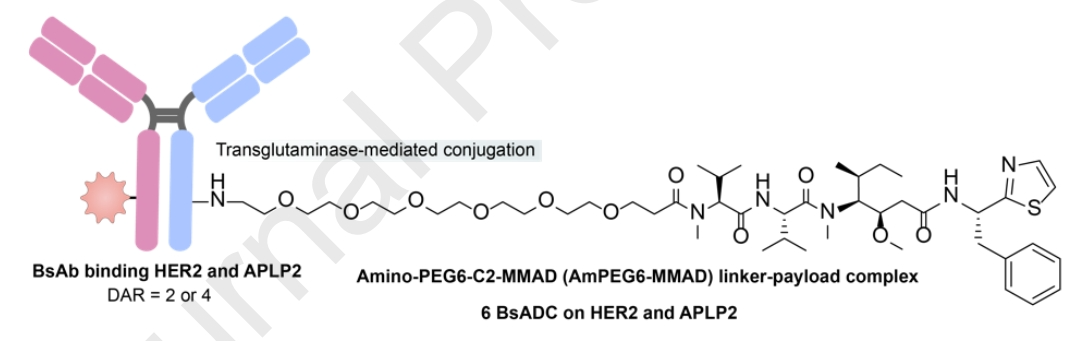
The intracellular tail of APLP2 contains overlapping tyrosine-based NPXY and YXXØ motifs. Following clathrin-mediated endocytosis, APLP2 can bind to AP-2, mediating effective internalization and directly leading to lysosomal degradation.
EGFR is a member of the ERBB receptor tyrosine kinase family and plays a key role in regulating fundamental functions of epithelial malignancies; however, due to treatment pressure induced acquired genomic alterations, targeting EGFR monoclonal antibodies and TKIs often leads to the emergence of clinical resistance. BsADCs are expected to address the mechanisms of anti-EGFR resistance, including sensitizing mutations and the activation of bypass pathways.
Targeting Dual-Epitopes of EGFR BsADCs
To mitigate the emergence of resistance, a bispecific antibody targeting two different epitopes of EGFR has been developed. This bispecific antibody is designed by fusing nanobodies specific to non-overlapping epitopes on EGFR (9G8 and 7D12).
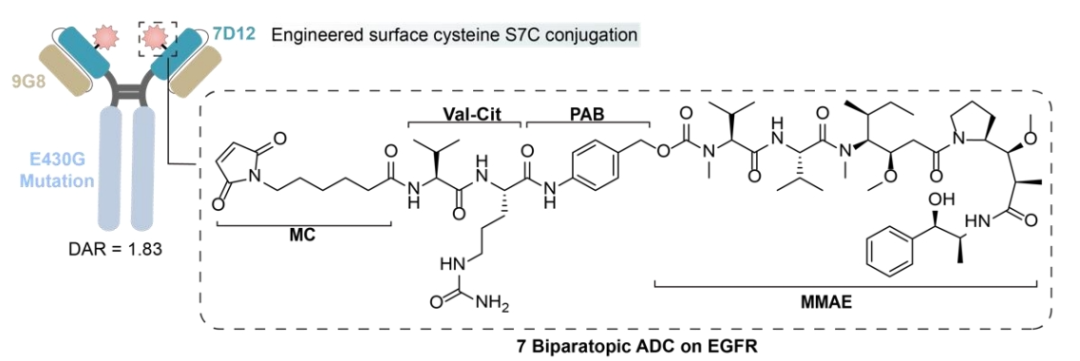
Among them, 7D12 disrupts the EGFR signaling cascade, while 9G8 stabilizes the bound conformation of EGFR-ECD, spatially preventing dimerization. Additionally, 7D12 and 9G8 exhibit potency against different EGFR mutant cell lines, inducing more effective CDC effects in cells expressing wild-type EGFR or exhibiting resistance mutations to cetuximab.
Targeting EGFR and Other Antigens BsADCs
BL-B01D1 is the first dual-antibody ADC in China to enter phase I clinical trials, targeting EGFR and HER3, with its proprietary Ac linker, which has better stability and hydrophilicity compared to Mc linker, and is less prone to aggregation; the toxin is a proprietary camptothecin analog ED04. Its phase I clinical safety is good, with no drug-related patient deaths reported. In 10 evaluable NSCLC end-line patients with good safety, the ORR was 60%, and the DCR was 90%.
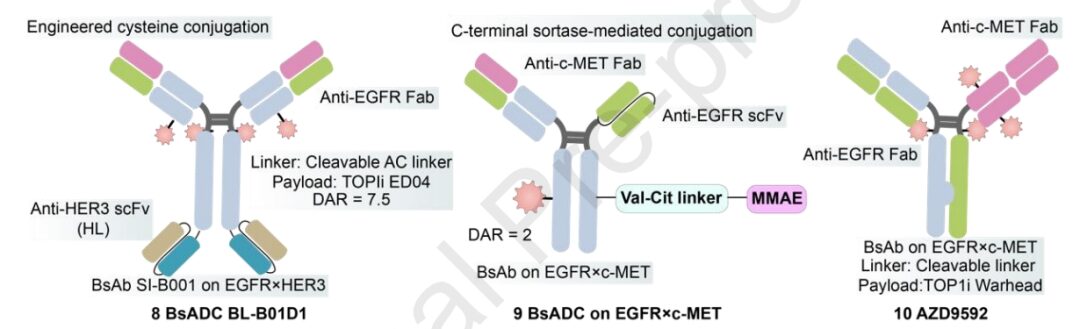
Currently, several BsAbs targeting c-MET and EGFR have been reported, demonstrating synergistic effects in inhibiting tumor proliferation and metastasis. In the design of BsADCs, careful selection of appropriate epitope combinations is crucial to avoid complete or partial disruption of c-MET. AZD9592 is an EGFR/c-Met ADC developed by AstraZeneca, conjugated with a novel topoisomerase I payload via a cleavable linker, primarily addressing osimertinib resistance. Compared to EGFR, AZD9592 has a higher affinity for c-MET, aiming to reduce normal tissue toxicity driven by EGFR. In PDX and resistant models, both monotherapy and combination with osimertinib exhibit good anti-tumor activity.
1231 is a MUC1/EGFR dual-antibody ADC developed in collaboration between Sutro and Merck subsidiary EMD Serono, employing non-natural amino acid site-specific coupling technology, conjugated with a cleavable VC linker hemiasterlin derivative (microtubule inhibitor), with a DAR of 4. Preclinical studies show strong anti-tumor activity in ESCC and NSCLC patient-derived xenograft models.
The hepatocyte growth factor (HGF)-mesenchymal-epithelial transition factor (MET) pathway plays a significant role in the development of various types and stages of cancer, from initiation to metastasis. Upregulation and amplification of MET are considered major escape routes during anti-EGFR therapy. C-MET can cross-react with EGFR, inducing resistance to EGFR-targeted therapies, making the inhibition of C-MET a viable strategy to overcome EGFR resistance.
Compared to traditional MET-targeting ADCs, the design of dual-epitope MET×MET-BsADCs offers innovative solutions to overcome existing challenges. MET bispecific antibodies have the ability to form 2:2 antigen-antibody complexes, promoting effective MET internalization and lysosomal transport. By conjugating the maytansinoid payload M114 via a protease-cleavable linker to the lysines on the surface of the BsAb targeting MET, REGN5093-M114 generates a BsADC with a DAR of 3.12.
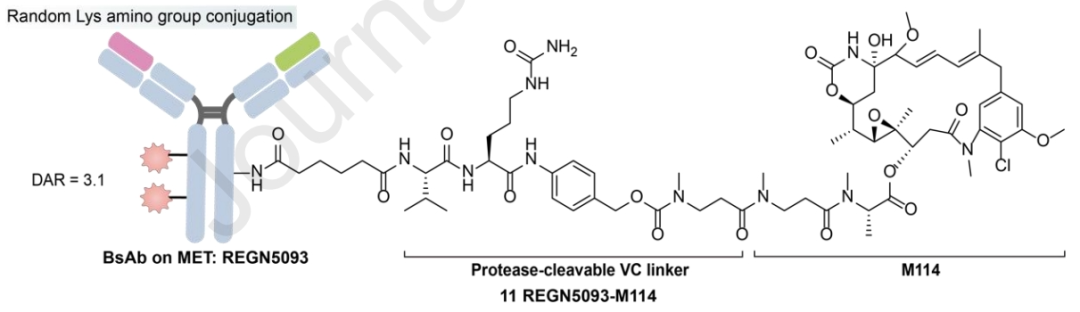
Preclinical data indicate that REGN5093-M114 significantly inhibits the proliferation of NSCLC cells overexpressing MET. It has currently initiated a phase I, dose-escalation, and expansion study to evaluate the safety and efficacy of REGN5093-M114 in adult patients with MET-overexpressing advanced cancers (NCT04982224).
BsADCs significantly expand the range of potential targets and scaffolds, beyond traditional paradigms. In fact, BsADCs provide an effective means to convert non-internalizing antigens into internalizing ones. For example, using EphA2, characterized by rapid internalization, and activated leukocyte adhesion molecule (ALCAM) (a non-internalizing or slowly internalizing antigen) to generate BsAb. Interestingly, when the ratio of cell surface EphA2 to ALCAM exceeds a threshold of 0.2, the bispecific antibody exhibits effective internalization. Conversely, when the ratio is below this threshold, internalization is hindered.
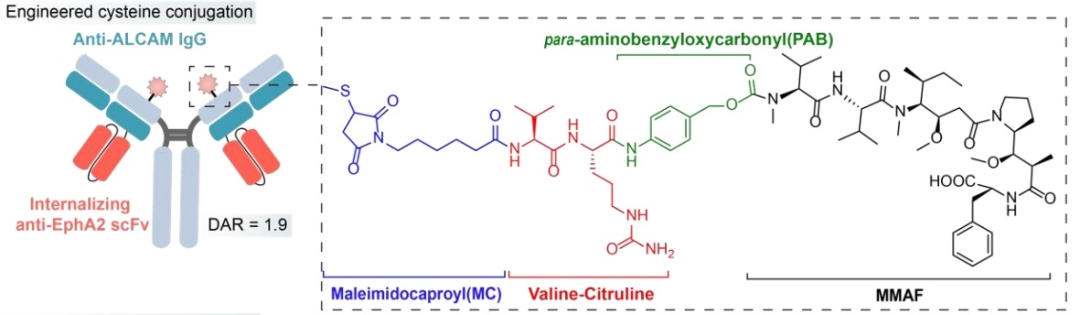
The MC-VC-pab-MMAF payload complex is site-specifically coupled via cysteine residue. In the context of dual-specific binding of BsADCs, the internalization effect can be significantly impacted by simultaneously targeting adjacent antigens, depending on their expression ratios. This ability broadens the range of antigen selection and can be used to design types of BsADCs with poor internalization. This innovative approach provides a subtle strategy to enhance the internalization potential of certain antigens, contributing to the multifunctionality and effectiveness of BsADCs targeting a broader range of tumor antigens.
The introduction of various new technologies has revolutionized the generation of BsADCs, presenting distinct advantages. Another significant advancement is PEG-conjugated BsADCs (P-BsADC), which not only ensure uniform conjugation but also exhibit high endocytosis efficiency, tissue penetration, and reduced toxicity due to their small molecular weight and absence of Fc fragments.
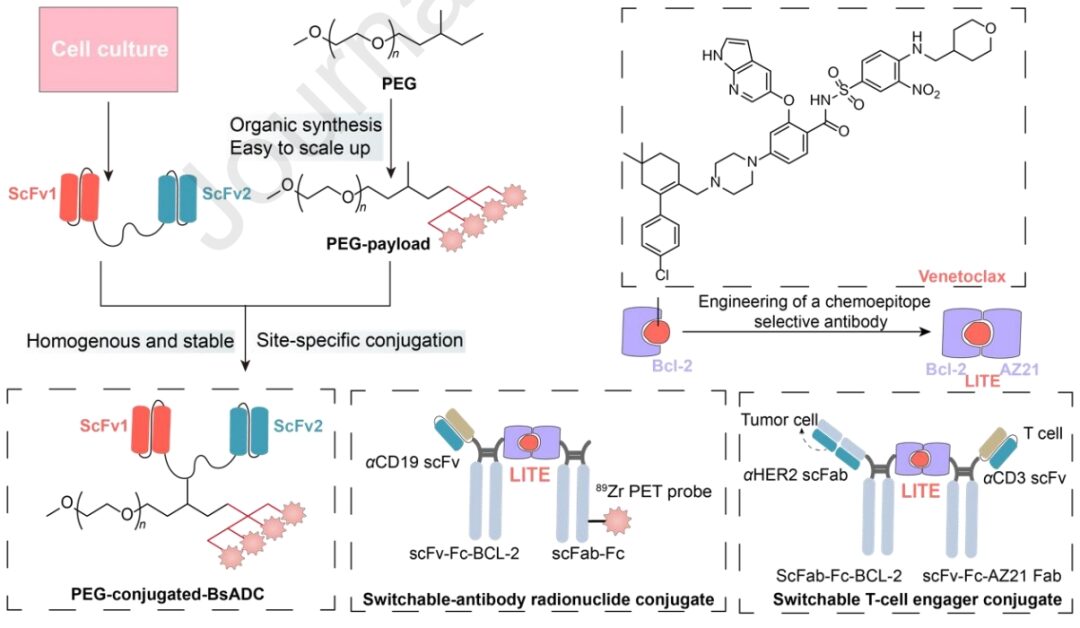
Additionally, ligand-induced transient binding of multiple antibody domains (LITE) is a cutting-edge technology that combines the advantages of extending the half-life of biopharmaceuticals with precise temporal control of small molecule-related activity. These innovative strategies hold great promise in maximizing therapeutic effects while minimizing off-target effects, representing an important step in the development of BsADC technology.
BsADCs represent a novel therapeutic category that merges the advantages of ADCs and BsAbs. However, challenges remain, primarily due to the complexity of solid tumors, including heterogeneity, histological barriers, and poor permeability. The design strategies of BsADCs must be refined to overcome these challenges.
Broadening Antibody Scaffolds
The current target selection of BsADCs is still somewhat limited, primarily focusing on HER2, c-MET, and EGFR. However, bispecific strategies have the potential to expand the range of targets, including those with poor internalization or low expression. Given the diversity of BsAbs, further enriching the antigen selection of BsADCs can diversify anti-tumor mechanisms. Promising avenues include immunomodulatory BsADCs based on T cell engagement or PD-L1 targeting.
Eliminating Heterogeneous Conjugation
Constructing BsADCs through random chemical coupling based on functional groups carries the risk of heterogeneous conjugation. This heterogeneity can disrupt the bispecific binding mode and alter the physical and PK characteristics of BsADCs. Site-specific coupling strategies can produce BsADCs with uniform DAR, ensuring not only the accuracy of drug delivery but also the consistency of therapeutic responses, making it a key consideration in the design and development of BsADCs. It is essential to emphasize that the foundation of site-specific coupling lies in the careful selection of coupling sites, a key factor affecting the efficacy of ADC drugs.
Clarifying Parameters Between Two Targets
The polyvalent binding mode and parameters of BsADCs impose mutual constraints and dependencies. Design considerations between the two targets need careful examination, particularly regarding variations in affinity, expression, and valency. The bispecific binding mode influences overall internalization not only through valency interactions but also through cross-arm binding. In addition to affinity, the expression threshold of surface antigens in cancer cell lines plays a critical role in determining overall drug efficacy. Addressing these key points requires comprehensive screening of combinations with different affinities of binding arms to achieve optimal bioactivity.
Transport
BsAbs can navigate by utilizing the initial specificity of the BsAb merely as a second specificity transport mode. An example is the BsAb platform that utilizes low-affinity transferrin receptor to carry β-secretase antibodies, facilitating effective passage through the blood-brain barrier. BsADCs targeting CD63, PRLR, and APLP2 have been reported to exhibit auxiliary transport capabilities. Based on these strategies, designing BsADCs is expected to proficiently traverse biological barriers and evade lysosomal degradation.
Addressing Potential Safety Issues
While BsADCs aim to enhance specificity, thereby alleviating off-target toxicity and side effects, early clinical data from ZW49, MEDI4276, and BL-B01D1 indicate that their clinical safety may not meet expectations. Off-target toxicity is a major feature of ADC-related toxicity. However, relying solely on BsAbs may not sufficiently address the reduction of off-target toxicity. In addition to antibody innovations, crucial research surrounding linker-payload complexes is also essential, including considerations of linker stability, homogeneous conjugation strategies, DAR, and bystander killing effects.
In conclusion, the emergence of unique dual-specific targeting patterns brings new innovations to the field of ADCs, marking the birth of a new generation of ADCs. Although still in the early stages of development, BsADCs offer a promising new approach. BsADCs are significant for overcoming existing clinical challenges faced by traditional ADCs and developing more precise targeted therapies.
In addition to BsADCs, a series of next-generation strategies are expected to make epoch-making contributions to the design of novel ADCs. These include ADCs with dual payloads, immunomodulatory ADCs, radionuclide ADCs, precursor ADCs, ADC combination therapies, and peptide-drug conjugates. Future assessments of these strategies in the application of novel ADCs, whether alone or in combination, will provide important prospects for advancing the development of this field.
References:
1. Bispecific antibody drug conjugates: Making 1+1>2. Acta Pharmaceutica Sinica B. 20 January 2024


