Design Tips
1
Microfluidic chip
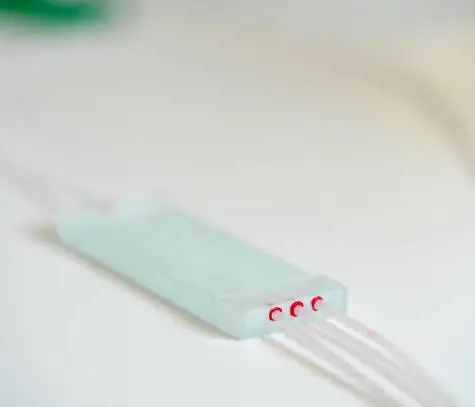
Since the development of the first commercial POCT instruments in the 1990s, microfluidic technology has gained increasing favor among manufacturers due to its outstanding advantages, especially in the field of diagnostics. Microfluidics is a branch of fluid mechanics that studies the physical processes of fluid flow using fundamental control equations, representing fluid science at the micron level. Microfluidic systems reduce the complexity and scale of experiments, providing powerful tools for the development of diagnostic instruments.
Currently, microfluidic technology has become a trend and is the preferred technology in the modern in vitro diagnostic industry.
Disposable chip consumables typically need to integrate complex functions in a simple manner while requiring the smallest possible volume, which often takes the form of high-precision microfluidic boxes, presenting some challenges for developers. Below, we outline some considerations to improve the success rate of projects in the early stages of microfluidic box development.
Design Requirements
Design Requirements


The development of detection instruments requires a thorough understanding of the reagents used and their properties, such as viscosity, chemical compatibility, and required volume. It is also important to understand how the reagents enter the microfluidic chip. Are they pre-loaded into the chip, or are they packaged in vesicles? The packaging type will affect the storage, effectiveness, and interactions with other reagents. Some microfluidic devices prefer lyophilized reagents to avoid complex liquid handling.
The fluid properties of the reagents can determine how fluids move within the storage areas and channels. It is advisable to create a detailed list of the features and processes involved in the analytical scheme, such as the number of reagents in the scheme, their volumes, fluid properties, volatility, material compatibility, biocompatibility, and components. At the same time, determine the driving method according to the requirements, which includes positive pressure, vacuum, electrostatics, ultrasound, capillary action, or fluid pumps, such as diaphragm pumps, piston pumps, membrane pumps, or even peristaltic pumps.
Driving time and flow rate are the most critical requirements. Considering that microfluidic processes are a miniaturized version of laboratory workflows, it is essential to time each process appropriately under the specified volume. Some detection processes include target analytical processes for each unit operation, such as sample introduction, mixing, metering, culturing, pre-filtration, separation, sorting, conjugation, and washing steps. We recommend listing all unit operations, along with the corresponding reagents, volumes, processes, times, flow rates, and other specific parameters.
Incorporating these considerations into the design requirements will guide engineers in conceptualizing the microfluidic chip, effectively implementing the detection scheme, while determining manufacturability and cost.
Conceptualization
Conceptualization
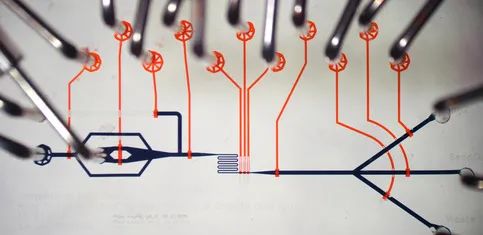
With clear requirements, a preliminary design concept can be drafted. If the design requires external components such as pumps, valves, filters, tubing, ports, and interfaces, the specifications of such components must be reviewed, considering their compatibility with the chip materials and the feasibility of integration into the chip. In most cases, external components drive the overall size and performance of the microfluidic box.
Other key elements of the initial chip design include unit operations performed within the chip, such as sample in and out, reagent storage, mixing, metering, material filtering, and analyte detection. Sample inlets and outlets are crucial for user interface design. The introduction and collection of samples determine the usability and human factors of the processing chip. Operators find poorly designed microfluidic chip consumables difficult to use, or in some cases, they may even pose various risks.
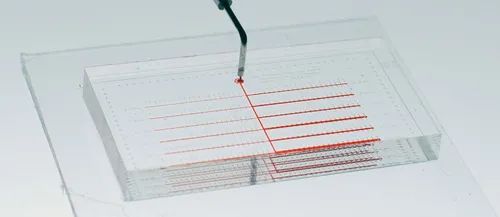
Elements in Chip Design:
Reagent Mixing in Microfluidic Chips – This includes mixing biological samples with other reagents (i.e., dissolving, labeling, recombining, diluting, and merging). Mixing within the chip can be done in various ways, such as shaking, magnetic mixing, using vortices, applying acoustic pressure, material transfer, or other mixing techniques implemented within the chamber.
Sample Metering – This is one of the most challenging functions to achieve in microfluidic boxes. Since microfluidic channels handle fluids in sub-microliter volumes, the accuracy of fluid metering is crucial. In detections requiring precise measurements, metering poses a significant challenge. Metering is generally divided into passive metering and active metering. Passive metering uses predetermined channel and reservoir volumes to dispense the required reagents. Active metering uses sensors to monitor the amount dispensed.
Material Selection – The polymer used for the chip must be tested for processability, biocompatibility, manufacturability, and cost. It is essential to consider the performance of the polymer, such as thermal performance, bonding, stability, and optical properties, with the final choice depending on analytical chemistry and biocompatibility. Manufacturability determines the yield and total cost of the final product.
Analyte Detection – This is particularly necessary for POCT devices and benchtop equipment. In some designs, optical lenses can be integrated into the chip to facilitate imaging or electronic data collection.
Assembly Process
Assembly Process
During and after the conceptualization of the chip box design, consideration should be given to how the microfluidic box will be assembled. In small batch production, this may include methods such as laser cutting, embossing, and micro-milling, which can be assembled using adhesives or other bonding techniques. To simplify the assembly process, it is essential to understand the assembly process as early as possible. Steps in the chip components include part manufacturing, cleaning, integration of external components, stacking, and quality control testing.
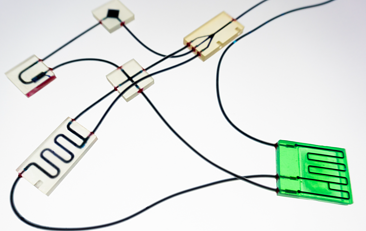
Assembly Fixtures and Jigs. Assembly fixtures and jigs are key considerations in the conceptualization and detailed design process of the chip. They depend on the dimensional tolerances of each layer and directly affect the tolerance stacking of multilayer chip configurations. Early consideration of these factors helps avoid assembly problem traps in early prototypes while simplifying chip box design and using larger channels where possible.
Quality Control Testing. Quality control checks should always be conducted on the design of the microfluidic box to ensure reliability and repeatability of performance. This includes checking dimensions within specified tolerances, as well as pressure decay testing and fluid testing to assess the functionality of the chip box.
Assembly Trial Runs. Trial runs of the assembly workflow provide debugging and troubleshooting of potential risks in the assembly process before production units, while making changes to improve assembly efficiency.
Detailed Design
Detailed Design
In the detailed design process of the chip box, designers must have a thorough understanding of the manufacturing processes to be used and conduct experiments and iterations on those processes to establish a set of rules that can ensure the quality of the manufacturing process.
Iterations in chip box design can prototype faster in a systematic process. Preliminary testing can provide feedback to optimize chip design, offering more detailed design parameters.
Quality Inspection and Testing
Quality Inspection and Testing
Microfluidic boxes are typically single-use. Setting good quality control objectives early in the prototype phase helps ensure the reliability and repeatability of prototypes, thereby improving production efficiency. Methods for monitoring quality issues related to chip box design include:

●Detailed functional quality checks. Detailed checks of chip characteristics, such as dimensions, surface finish, cleanliness, and integration of external components. Preliminary inspections can be used to screen key characteristics in quality control checks during production.
●Potential failure modes of components. Conducting assembly failure mode analysis early in the chip box prototype design to ensure successful assembly of the chip box and avoid issues such as misaligned parts and leaks due to improper bonding and integration of external components during assembly.
●Inspection of critical components and channel profile. Based on detailed inspection results and design specifications, critical characteristics should be identified and inspected as part of the quality control process. Use a profilometer to check channel profiles to ensure surface roughness and channel depth are within specification ranges, as they affect fluid flow parameters within the chip.
●Pressure decay tests. Pressure decay testing sets benchmarks for the successful sealing of microfluidic boxes.
●Fluid flow testing. To assess the performance of the chip, colored reagents are used during fluid flow testing. Using colored water helps visually observe the tested chip box and can effectively evaluate the mechanical performance of the product.
The development of microfluidic chips is a complex process. Using clever methods to advance the development process helps reduce risks associated with microfluidic chip development, thereby improving product success rates.
【The above text is from the WeChat public account ‘ITL Innovative Instrument Development’, reprinting is not allowed. For authorization, please contact the original author.】
Previous Recommendations
Microfluidics and Fluid Mechanics
A post about ‘Rapid Nucleic Acid POCT Integrated Microfluidics’ patent study sharing
Nucleic Acid Testing POCT Product Study
Important Systems for Controlling Fluids in Microfluidic Chips
More Exciting Content
Follow Xiao Qian, click on ‘Knowledge Points’ and ‘Work Topics’ in the chat box to learn more! Welcome to leave a message~


Code has “Xiao Qian”