AI Summary
- The patient had platinum-resistant ovarian cancer with multiple treatment failures, presenting with liver, lymph node, and bone metastases, and conventional treatments were ineffective.
- After using the novel targeted drug RC88, significant shrinkage of liver metastases and hilar lymph nodes was observed, with a reduction in the extent of bone metastases, achieving partial remission.
- The main side effects during treatment were leukopenia, neutropenia, and anemia, which were manageable with symptomatic treatment.
- RC88 targets the tumor marker MSLN (mesothelin), which is highly expressed in ovarian cancer, making it particularly suitable for patients with positive detection.
- Options for treating platinum-resistant ovarian cancer are limited, and RC88 offers new hope for patients who have failed multiple lines of treatment.
- Clinical trials show that RC88 has a tumor shrinkage rate of approximately 41.7% in MSLN-positive patients, with a disease control rate exceeding 90%.
- Regular monitoring of blood counts and liver function is required during treatment to manage side effects such as bone marrow suppression.
- Patients with bone metastases can use bisphosphonates like zoledronic acid in conjunction with RC88 treatment.
- The drug is still in clinical trials and requires further validation of long-term efficacy and safety, but early results are promising.
Platinum-resistant ovarian cancer (PROC) is a common stage in the progression of ovarian cancer, with generally poor prognosis for patients. Current conventional treatment methods are ineffective, highlighting the urgent need for new therapeutic drugs. This article reports a case of a PROC patient who progressed after multiple lines of treatment and received the novel antibody-drug conjugate (ADC) RC88 targeting mesothelin (MSLN) in a clinical trial. After treatment, significant shrinkage of liver metastases and hilar lymph nodes was observed, along with a reduction in the extent of bone metastases, achieving partial remission. This report summarizes the patient’s medical history, imaging findings, genetic testing, treatment regimen, and outcomes, and reviews the treatment methods for PROC, exploring the application prospects of RC88 as a new drug in PROC treatment. Targeting MSLN, RC88 shows good anti-tumor effects and safety in PROC treatment, significantly reducing tumor burden and providing new hope for advanced PROC treatment.
0 Introduction
Platinum-based drugs are among the most effective treatments for ovarian cancer, with initial response rates as high as 80%. However, platinum resistance is inevitable as the disease progresses. Platinum-resistant ovarian cancer (PROC) refers to disease progression within six months after the last platinum-based chemotherapy, which is very common in clinical practice, as nearly all recurrent ovarian cancer patients eventually experience platinum-resistant recurrence [1-2]. Currently, treatment for PROC mainly relies on non-platinum chemotherapy drugs and anti-angiogenic targeted therapies, but these methods have suboptimal efficacy, and patient prognosis remains poor, failing to meet clinical treatment needs [3]. This patient experienced disease progression after surgery, chemotherapy, immunotherapy, targeted therapy, and radiotherapy, assessed as platinum-resistant recurrence characterized by multiple liver, lymph node, and bone metastases, with conventional drug treatment unable to control disease progression. The tumor tissue showed strong positivity for mesothelin (MSLN). After treatment with the injectable antibody-drug conjugate (ADC) RC88, measurable lesions including liver metastases and hilar lymph nodes showed significant shrinkage, and the extent of bone metastases also decreased, demonstrating significant clinical efficacy, as reported below.
1 Clinical Case
The patient is a 46-year-old female who presented to the gynecology department of Nanjing University Medical School Affiliated Gulou Hospital in August 2016 due to “abdominal distension for 2 weeks,” where abdominal effusion was found, and cytological examination revealed cancer cells. On September 1, 2016, she underwent “ovarian cancer tumor debulking surgery (hysterectomy + bilateral salpingo-oophorectomy + appendectomy + omentectomy).” Postoperative pathology confirmed high-grade serous adenocarcinoma of the bilateral ovaries. The left ovarian mass measured 3 cm × 2 cm × 1 cm, with cancerous tissue infiltrating and penetrating the corresponding ovarian capsule, with no nerve invasion observed, and cancer thrombus present in blood vessels; the right ovarian mass measured 2.5 cm × 1.8 cm × 1.2 cm, with similar findings. No cancerous tissue was found in the bilateral fallopian tubes, their walls, or the uterine wall; the cervical margin showed no cancerous tissue; the endometrium showed proliferative changes, and the cervix showed chronic cervicitis; atypical tissue was noted as proliferative fibrous granulation tissue with focal cancer infiltration; chronic appendicitis was also noted, with adenocarcinoma infiltration in the omentum. Immunohistochemical results showed that cancer cells expressed: ER (weak +), PAX8 (+), p53 (-), CK7 (+++), CA125 (+++), CK20 (-), Ki67 approximately 40% (+), CK5/6 (-), Vim (-), PR (-), p63 (-), WT-1 (+); follicular cyst covering cells expressed p63 (-). The surgical pathological stage was classified as stage IIIc. On September 7, 2016, she received one cycle of chemotherapy with liposomal paclitaxel (210 mg) intravenously and cisplatin (100 mg) intraperitoneally. From September 29, 2016, to January 16, 2017, she underwent six cycles of chemotherapy with liposomal paclitaxel (210 mg) and carboplatin (600 mg) intravenously.
In April 2018, a follow-up ultrasound showed liver lesions, and further CT and MRI examinations indicated a lesion at the top of the liver diaphragm, suspected to be a metastatic focus, classified as platinum-sensitive recurrence. From April 28, 2018, to September 7, 2018, she received six cycles of chemotherapy with gemcitabine (1.6 g d1, d8), cisplatin (50 mg d1~3), and bevacizumab (500 mg d0), with a partial response (PR) evaluated. Subsequently, she received maintenance treatment with bevacizumab (500 mg) until May 9, 2019, during which hereditary tumor 55-gene whole exome testing indicated a significant but unclear SMARCA4 mutation, with no significant pathogenic mutations found in BRCA1/2.
On April 15, 2021, a follow-up abdominal CT showed enlarged nodules at the top of the liver diaphragm and in the liver-kidney space, along with multiple enlarged lymph nodes in the retroperitoneum. On May 4, 2021, serum CA125 was 50.07 U/mL, indicating disease progression (PD) and another platinum-sensitive recurrence. From May 9, 2021, to October 11, 2021, she underwent six cycles of intravenous chemotherapy with albumin-bound paclitaxel (300 mg d1) and nedaplatin (40 mg d1~2), with a PR evaluated. From November 5, 2021, to February 2023, she received maintenance treatment with oral olaparib (300 mg bid).
On February 14, 2023, a follow-up CT indicated enlarged retroperitoneal lymph nodes, suggesting PD. From April 11, 2023, to May 10, 2023, she underwent radiotherapy for retroperitoneal lymph nodes, completing a dose of PTV 2 Gy × 20 f, PGTV 3 Gy × 20 f, during which she received four cycles of concurrent irinotecan chemotherapy. A follow-up CT on May 23, 2023, showed significant shrinkage of retroperitoneal lymph nodes, with a PR evaluated. From May 26, 2023, to August 9, 2023, she underwent four cycles of chemotherapy with irinotecan (200 mg d1) and cisplatin (40 mg d1~2). A follow-up CT and MRI on September 9, 2023, indicated multiple lesions in the liver, suspected metastatic lesions, and multiple enlarged lymph nodes at the hepatic hilum, assessed as platinum-resistant recurrence.
On September 15, 2023, a liver lesion biopsy was performed, and pathology indicated metastatic tumors, likely of ovarian origin, with positive MSLN detection (Figure 1). The patient voluntarily participated in a multicenter, open-label, multi-cohort phase I/IIa clinical study evaluating the safety, efficacy, and pharmacokinetics of the injectable novel MSLN-targeted ADC RC88 in patients with advanced malignant solid tumors (Study No.: RC88-C001). From October 9, 2023, to December 15, 2023, she underwent four cycles of RC88 treatment (dose group: 2.0 mg/kg, with doses of 128 mg in the first cycle, 130 mg in the second cycle, 126 mg in the third cycle, and 124 mg in the fourth cycle); follow-up CT after the second and fourth cycles indicated significant shrinkage of measurable lesions in the liver and hilar lymph nodes, achieving a PR. A baseline ECT whole-body bone scan on September 25, 2023, showed no significant abnormalities. A bone scan on November 21, 2023, suggested possible bone metastases, with MRI confirming multiple metastases in the right accessory area of T5, L5 vertebra, bilateral iliac bones, and sacrum, with imaging analysis not ruling out the possibility of baseline bone metastases. On December 5, 2023, zoledronic acid protection therapy was initiated (28 d/cycle) in conjunction with RC88 treatment. A follow-up MRI on December 30, 2023, showed a reduction in the extent of bone metastases compared to previous imaging (Figure 1). During treatment, the patient experienced leukopenia, neutropenia, anemia, mild appetite loss, mild elevation of alanine aminotransferase, and finger numbness as adverse reactions. After symptomatic treatment and careful management, these symptoms were effectively controlled or resolved.
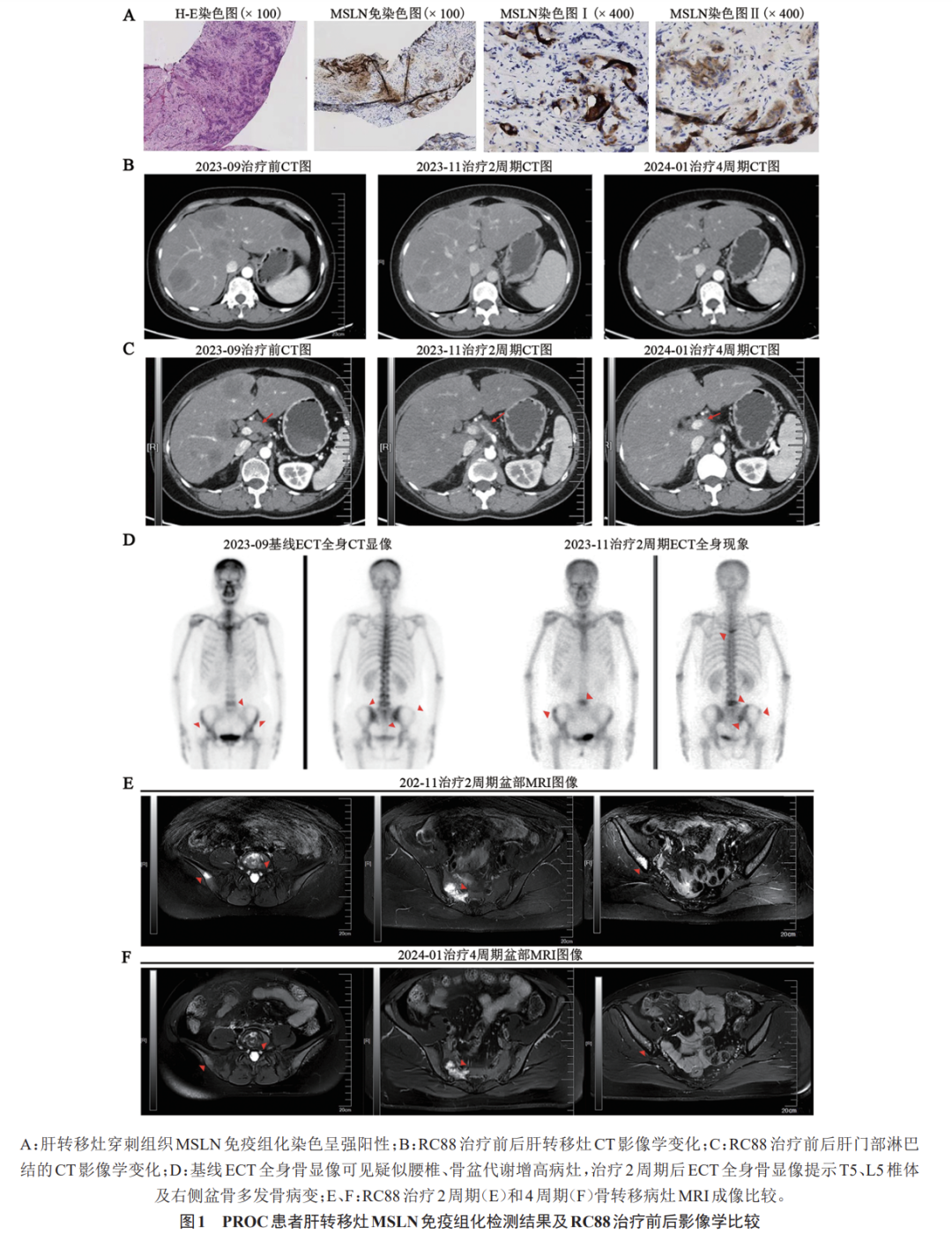
2 Discussion
Ovarian cancer is one of the malignant tumors that severely affect women’s health. In 2022, approximately 313,959 women were diagnosed with ovarian cancer globally, with 207,252 deaths [4]. Due to the atypical early clinical symptoms and lack of effective screening methods, most ovarian cancer patients are diagnosed at an advanced stage of the disease [5]. Despite surgical resection combined with standardized chemotherapy, most ovarian cancer patients still experience recurrent relapses and disease progression, with the platinum-free interval (PFI) continuously shortening, leading to all platinum-sensitive recurrent ovarian cancers eventually evolving into PROC [6]. The treatment efficacy for PROC is low, and patient prognosis is poor. In recent years, although the short-term efficacy of targeted drugs such as poly (ADP-ribose) polymerase inhibitors (PARPi) and bevacizumab has improved somewhat, this challenge remains unresolved [7-9], and the efficacy of immune checkpoint inhibitors in PROC has been disappointing [10]. In recent clinical studies, the novel ADC mirvetuximab soravtansine targeting folate receptor alpha (FDα) has shown good efficacy in PROC patients, with an objective response rate (ORR) of 32.4% [95% CI (23.6, 42.2)], and a median duration of response of 6.9 months [95% CI (5.6, 9.7)], which has been approved by the FDA for the treatment of FRα-positive PROC [8,11].
ADCs combine the high specificity of monoclonal antibody drugs with the high activity of small-molecule cytotoxic drugs, which not only enhances the targeting of tumor drugs but also reduces toxic side effects [12]. MSLN is a glycosylphosphatidylinositol-anchored cell surface glycoprotein that is only expressed in mesothelial cells within the pleura, peritoneum, and pericardium in normal human tissues, but is overexpressed in some solid tumor cells, with expression rates of 60%-65% in ovarian cancer, and even up to 97% in high-grade serous ovarian cancer [13]. Some studies [14-15] suggest that MSLN, along with CA125, participates in the proliferation, adhesion, and metastasis of ovarian cancer cells, closely related to the drug resistance of ovarian cancer cells, and downregulation of MSLN expression can partially reverse cell resistance and reduce the toxicity of chemotherapy drugs, making MSLN a promising target for targeted therapy in ovarian cancer. Injectable RC88 is a novel anti-MSLN ADC formed by conjugating a recombinant humanized MSLN monoclonal antibody with the microtubule inhibitor monomethyl auristatin E (MMAE). RC88 has a high affinity for MSLN, specifically binding to tissues expressing MSLN, competing with endogenous ligands, and exhibiting cytotoxic effects on tumor cells with varying levels of MSLN expression, with its cytotoxicity positively correlated with MSLN expression. Preclinical studies [16] have shown that RC88 exhibits significant tumor inhibition in a mouse model of human ovarian cancer OVCAR-3 cells. Results from a multicenter, non-randomized, open-label phase I/IIa clinical study (RC88-C001, RC88-C002) initiated in 2020 (Clinical Trial Registration No.: CTR20192142) indicated that the ORR in the ovarian cancer cohort with ≤4 prior lines of treatment was 41.7%, with a disease control rate (DCR) of 91.7%. The most common related adverse reactions were hematological toxicity, gastrointestinal toxicity, and elevated transaminases, all of which were manageable, demonstrating a favorable benefit-risk ratio. Additionally, RC88 has also shown good anti-tumor activity in the treatment of cervical cancer and lung cancer. Currently, a single-arm, multicenter phase II clinical study is underway based on these findings, aiming to include PROC, fallopian cancer, and primary peritoneal cancer subjects who have relapsed after 13 lines of systemic anti-tumor treatment to clarify the efficacy and safety of RC88 monotherapy, with results still under evaluation.
This patient was already diagnosed with advanced high-grade serous ovarian adenocarcinoma at initial treatment. Despite undergoing surgical treatment and standardized chemotherapy, targeted therapy, maintenance therapy, and local radiotherapy, she experienced multiple recurrences and ultimately progressed to PROC after multiple lines of treatment, characterized by rapid disease progression with multiple liver, lymph node, and bone metastases, and a high tumor burden that conventional drugs could not control. The MSLN detection in her tumor tissue was strongly positive. After treatment with injectable RC88, significant shrinkage of measurable liver metastases and hilar lymph nodes was observed, along with a reduction in the extent of bone metastases, demonstrating significant clinical efficacy. The main adverse reactions experienced by the patient after treatment were bone marrow suppression (grade II leukopenia, grade III neutropenia, anemia), which is considered related to the toxicity of MMAE on hematopoietic cells in the bone marrow [17]. This toxicity is easily monitored clinically and can be alleviated and improved with appropriate medications, while other adverse reactions remained within manageable limits, indicating overall good safety and tolerability.
As a novel MSLN-targeted ADC, RC88 may represent a promising therapeutic strategy for PROC, demonstrating significant anti-tumor activity and good safety and tolerability in PROC treatment. However, current clinical trials have not systematically included assessments of patients’ immune function, which affects the comprehensive evaluation of how the drug impacts the immune system. Therefore, more multicenter, randomized clinical trials are needed in the future to validate the efficacy and safety of RC88.
Source
Yin Zi, Deng Liping, Zhou Fan, et al. Treatment of Platinum-Resistant Ovarian Cancer Progressing After Multiple Lines of Treatment with a Novel Antibody-Drug Conjugate: A Case Report and Literature Review. Chinese Journal of Cancer Biological Therapy, 2025, 32(1): 115-118. DOI:10.3872/j.issn.1007-385X.2025.01.016.
References omitted