
Introduction
The DS8201-A-U105 study reveals the latest advancements in ADC combined immunotherapy. What insights can we draw from it?
The annual European Society for Medical Oncology Breast Cancer (ESMO BC) conference was officially held from May 3-5, 2022, reporting a series of significant advancements in the field of breast cancer. ADCs have become star drugs in breast cancer treatment in recent years, and related research has naturally attracted much attention. Among them, a major analysis result of the DS8201-A-U105 study (abstract number: 162O) was presented in an oral report session, showcasing new progress in the treatment model combining ADCs with immune checkpoint inhibitors. We are honored to invite Professor Liu Yunjian from the Fourth Hospital of Hebei Medical University to provide an in-depth interpretation of these research findings.

Introduction to the DS8201-A-U105 Trial
The DS8201-A-U105 trial (NCT03523572) is a 2-part, open-label, phase 1B clinical trial assessing the combination of DS-8201 and nivolumab in patients with HER2-expressing advanced breast cancer. Part 1 involves dose escalation, while Part 2 is a dose expansion; Part 2 includes cohort 1 and cohort 2, which enrolled 29 HER2-positive (IHC 3+ or IHC 2+/ISH+) patients previously treated with T-DM1 and 16 HER2-low expressing (IHC 1+ or IHC 2+/ISH-) patients who progressed after standard treatment. The primary endpoint of Part 1 is the maximum tolerated dose (MTD) or recommended expansion dose (RDE), while the primary endpoint of Part 2 is the objective response rate (ORR) assessed by independent central review (ICR).
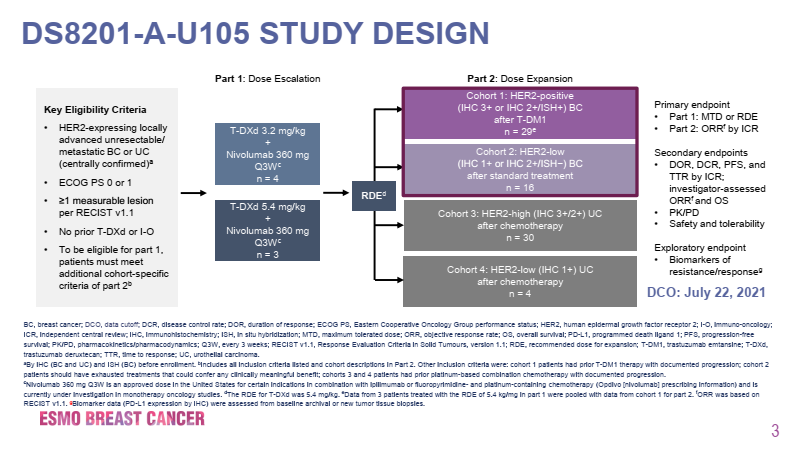
Figure 1. Design of the DS8201-A-U105 Trial
As early as the 2020 SABCS conference, interim analysis data of this study was reported[2]. The median follow-up time for the HER2-positive group was 7.0 months, while for the HER2-low expressing group it was 6.9 months, with ORRs of 59% and 38% respectively, and neither group reached the median duration of response (DOR). Most patients showed a reduction in the sum of target lesion diameters from baseline, with PFS of 8.6 months and 6.3 months respectively. In terms of safety, 43.8% of patients experienced treatment-emergent adverse events (TEAEs) of grade ≥3. Among all patients receiving RDE (n=48), the most common TEAEs of any grade were nausea (54.2%), fatigue (45.8%), and alopecia (41.7%).
The results announced at this conference showed that the median number of prior treatment lines for the HER2-positive group was 5, while for the HER2-low expressing group it was 4, with ORR benefits of 65.6% and 50.0% respectively, and CR rates of 9.4% and 0%. The median DORs for the two cohorts were not reached and 5.5 months respectively, with median PFS of 11.6 months and 7.0 months, and median OS of not reached and 19.5 months respectively. Overall, the safety profile was similar to previous reports of DS-8201 monotherapy, and the combination with nivolumab did not increase toxicity. 50.0% of patients experienced treatment-related adverse events of grade ≥3, with 51.7% in the HER2-positive group (34.5% related to DS-8201, 27.6% related to nivolumab) and 43.7% in the HER2-low expressing group (6.3% related to DS-8201, 18.8% related to nivolumab). The incidence of any grade interstitial lung disease (ILD)/pneumonitis was 14.6%, mostly grade 2, with an incidence of 12.5%, and only one case of grade 2 ILD/pneumonitis occurred in the HER2-low expressing group. A total of 6.9% of patients experienced left ventricular dysfunction (excluding the HER2-low expressing group). Exploratory biomarker analysis showed that the combination of DS-8201 and nivolumab was effective in both HER2-positive and HER2-low expressing breast cancer patients, regardless of PD-L1 IHC status.
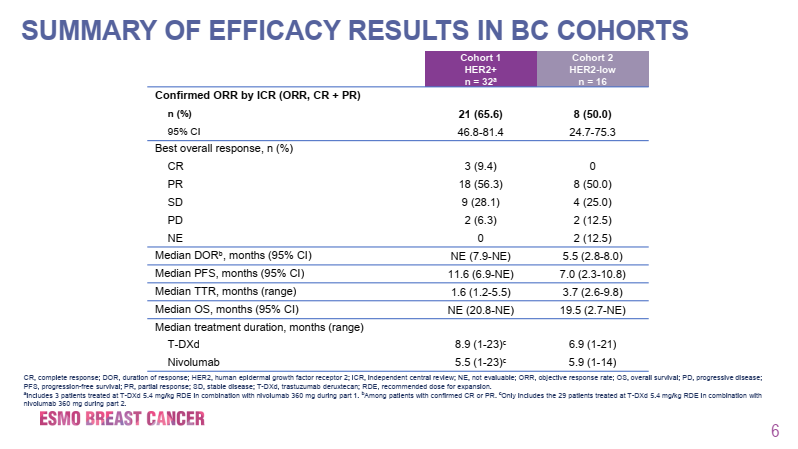
Figure 2. Patient Efficacy Results in the DS8201-A-U105 Trial
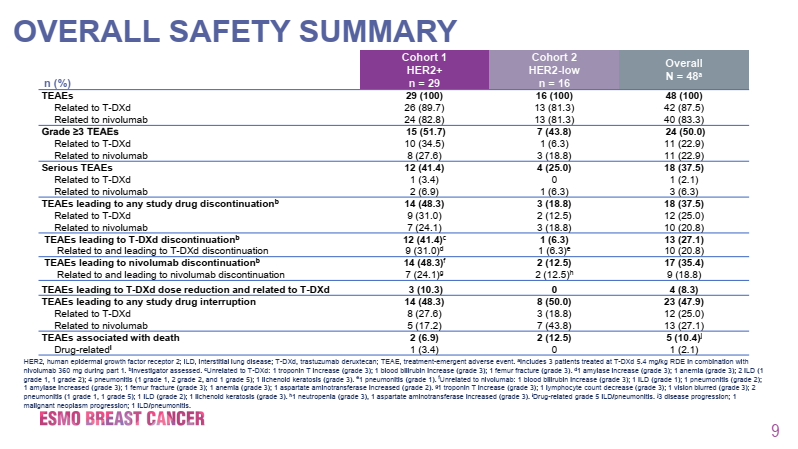
Figure 3. Overall Safety Data in the DS8201-A-U105 Trial
DS-8201 Combined with Immunotherapy Shows Efficacy Unaffected by PD-L1 Expression Status, Benefiting a Broader Population
Notably, exploratory biomarker analysis from the DS8201-A-U105 study indicates that the efficacy of DS-8201 combined with nivolumab is not affected by PD-L1 expression status, which may be related to the unique structure and mechanism of action of DS-8201. Its efficacy primarily relies on the release of highly active drug after endocytosis into tumor cells. When PD-L1 is highly expressed, the effect of immunotherapy is more pronounced; however, when PD-L1 is lowly expressed, the effect of immunotherapy may be diminished. Thanks to the high drug-antibody ratio (DAR=8) of DS-8201, it ensures a higher concentration of effective drug, exerting a strong cytotoxic effect on both targeted and adjacent tumor cells, significantly enhancing anti-tumor efficacy. This suggests that DS-8201 combined with immunotherapy has a broader beneficiary population.
Results of the DS-8201-A-U105 Study Should Not Be Overinterpreted; The Role of DS-8201 Combined with Immunotherapy Remains Undetermined
Compared to the data from the DESTINY-Breast01 and DS8201-A-J101 studies, the results of the DS8201-A-U105 study seem to indicate that the combination of nivolumab with DS-8201 does not further improve treatment benefits for HER2-expressing advanced breast cancer. However, regarding this result, Associate Professor Aditya Bardia from Massachusetts General Hospital in Boston stated that one should not overinterpret the results from this phase I clinical trial, especially regarding the comparison of efficacy. The role of immunotherapy combined with DS-8201 remains undetermined. Further research is needed to understand the roles of different drug carriers in mediating neoantigen generation and immune triggering, aiding in the subsequent development of more rational ADC drug and immunotherapy combinations.
ADC and Immunotherapy Have Synergistic Mechanisms, Providing Scientific Basis for Their Combined Application
An important background for conducting the DS8201-A-U105 study is that previous preclinical studies conducted in HER2-expressing tumor mouse models found that the combination of DS-8201 and anti-PD-1 antibody exhibited greater anti-tumor effects than either monotherapy[3]. Additionally, the rationale for combining ADCs with immunotherapy is supported by multiple preclinical and clinical observational results[4].
1
Studies using mouse models indicate that the activity of anti-HER2 monoclonal antibodies depends on cytotoxic T cells and interferon secretion, which can be improved by increasing anti-PD-1 blockade.
2
Another murine model study showed that anti-CTLA-4 antibodies can enhance the anti-tumor immunity induced by DS-8201.
3
Anti-HER2 monoclonal antibodies induce tumor regression not only through antibody-dependent cellular cytotoxicity (ADCC) but also through T cell-dependent mechanisms. ADCC induced by trastuzumab (or other trastuzumab-based ADCs) may lead to the activation of the adaptive immune system.
4
Evidence suggests that microtubule inhibitors and topoisomerase I inhibitors, which can serve as ADC payloads, can directly activate dendritic cells and stimulate their maturation. This immune activation may synergize with immune checkpoint inhibitors.
5
In an in situ tumor model expressing HER2 with primary resistance to immunotherapy, T-DM1 combined with anti-CTLA-4/PD-1 treatment was effective.
6
Tumor cell death may occur through several mechanisms, including apoptosis and autophagy, which may not elicit a significant immune response. In contrast, immunogenic cell death (ICD) of tumor cells mediates a regulated adaptive immune response through the release of damage-associated molecular patterns (DAMPs), leading to dendritic cell activation, recruitment of antigen-presenting cells, and activation of anti-tumor T cells. Chemotherapeutic agents such as anthracyclines, cyclophosphamide, oxaliplatin, and paclitaxel may induce ICD, and oncolytic viruses and peptides may also induce ICD. Increasing preclinical and clinical evidence suggests that ICD exhibits synergistic effects with PD-1 and PD-L1 inhibitors. Considering that ICD and amplified T cell responses are also induced by ADC payloads (such as DM1, MMAE, auristatin), there is reason to combine these ADCs with ICIs.
7
Some cytotoxic payload ADCs induce increased expression of major histocompatibility complex class I (MHC1) on tumor cells, which is highly relevant given that MHC1 loss is a significant mechanism of tumor evasion of T cell-mediated immune responses. Additionally, enhanced PD-L1 expression on tumor cells has also been reported.
8
In addition to their direct cytotoxic effects, some chemotherapeutic agents also possess inherent immune-stimulating properties, increasing the antigenicity and adjuvanticity of dying cancer cells, as well as leading to off-target effects that enhance the function of effector cells such as dendritic cells, CD4+ and CD8+ T cells, natural killer cells, and M1 macrophages, while reducing the activity of suppressive cells such as Tregs, M2 macrophages, and myeloid-derived suppressor cells.
9
Intrinsic or acquired resistance to ADCs may arise through various mechanisms, including downregulation of the monoclonal antibody target, receptor heterogeneity, truncation or masking of the target antigen, upregulation of efflux pumps, defects in internalization and endocytosis, lysosomal dysfunction, and defective cyclin B1. Resistance to PD-1/PD-L1 blockers may also be mediated by various mechanisms, including a lack of T cell infiltration in the tumor stroma, T cell exclusion, T cell dysfunction, disruption of antigen presentation, and resistance to interferons. Therefore, ADC combined with ICIs may help overcome resistance to either monotherapy.
10
Immune checkpoint inhibitors combined with targeted therapies such as trastuzumab can also reverse some patients’ resistance to targeted therapy. Studies have found that in PD-L1 positive, trastuzumab-resistant, HER2-positive advanced breast cancer patients, the combination of pembrolizumab and trastuzumab is safe and effective, with durable clinical benefits. Therefore, monoclonal antibodies that are part of ADCs may demonstrate synergistic effects with PD-1/PD-L1 inhibitors.
11
Increasing evidence suggests that the simultaneous use of immune checkpoint inhibitors and chemotherapy yields better clinical outcomes than using either alone. This dual therapy has become the standard treatment for patients with advanced non-small cell lung cancer and head and neck squamous cell carcinoma. A meta-analysis of immune checkpoint inhibitor treatment in advanced breast cancer patients showed that the ORR achieved when combined with other systemic therapies was higher than that of immune checkpoint inhibitors alone (26% vs. 9%). However, some common toxicities of cytotoxic chemotherapy (especially lymphopenia) may negatively impact the efficacy of immune checkpoint inhibitors, leading to worse outcomes. Considering that the incidence of lymphopenia with ADCs is lower compared to traditional chemotherapy, the payload delivered by ADCs may represent a better cytotoxic drug combination for use with immune checkpoint inhibitors.
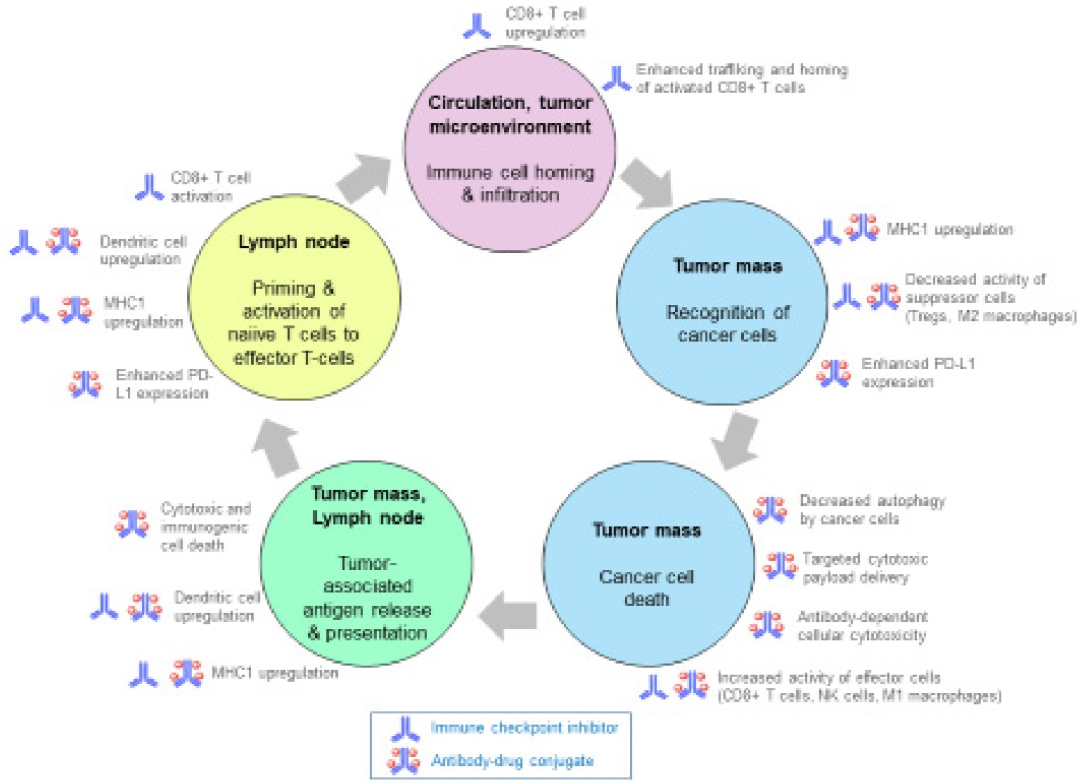
Figure 4. Synergistic Mechanisms Between ADCs and Immune Checkpoint Inhibitors
Active Exploration of ADC Combined Immunotherapy in Breast Cancer, More Results to Look Forward To
In addition to the DS-8201-A-U105 study, DS-8201 is also actively exploring combined immunotherapy in other studies. For example, the BEGONIA study cohort 6, which combines DS-8201 with PD-L1 inhibitors for first-line treatment of HER2-low expressing advanced TNBC, reported results at the 2021 ASCO conference (abstract number: 1023)[5], with a median follow-up time of 2.3 months, confirming an ORR of 4/4 (100%; only 4 patients had the opportunity to complete disease assessment during treatment), and all 4 patients responded by the data cutoff (median DOR not reached), with good safety data. The DESTINY-Breast07 study is an open-label, multicenter, dose exploration and expansion study assessing the safety and preliminary anti-tumor activity of DS-8201 as a monotherapy or in combination for HER2-positive advanced or metastatic breast cancer patients, including those with stable and active brain metastases. The DESTINY-Breast08 study is also exploring the application of DS-8201 combined with durvalumab in HER2-low expressing breast cancer. Additionally, studies combining DS-8201 with pembrolizumab for HER2-positive and HER2-low expressing breast cancer are actively underway (NCT03523572)[6].
In a small phase Ib study[7], advanced HER2-positive breast cancer patients who had previously received trastuzumab and paclitaxel were treated with T-DM1 combined with pembrolizumab, showing an ORR of 29% in 7 patients with PD-L1 CPS ≥1 and a median PFS of 2.9 months, while 6 patients with PD-L1 CPS <1 had an ORR of 33% and a median PFS of 8.7 months. In another phase Ib study[8], a cohort of HER2-positive advanced breast cancer patients received atezolizumab combined with T-DM1 or trastuzumab and pertuzumab treatment, with no new safety signals detected.
The KATE2 clinical trial is the first randomized, double-blind phase II clinical study exploring ADC combined with immunotherapy[9], enrolling 202 HER2-positive advanced breast cancer patients who had previously received trastuzumab and paclitaxel, randomly assigned (2:1) to the T-DM1 plus atezolizumab group (n=133) or the placebo group (n=69). There was no significant difference in median PFS between the two groups (8.2 months vs. 6.8 months, HR=0.82, 95% CI 0.55-1.23; p=0.33). In the PD-L1 positive subgroup, the median PFS for the two groups was 8.5 months and 4.1 months respectively (HR=0.60, 95% CI 0.32-1.11; p=0.099). The authors of this study suggested optimizing the selection of PD-L1 positive, HER2-positive disease patient subgroups most likely to benefit from this combination therapy. KATE3 (NCT04740918)[6] is an ongoing randomized, multicenter, double-blind, phase III clinical study aimed at evaluating the efficacy and safety of T-DM1 combined with atezolizumab or placebo in patients with unresectable locally advanced/MBC HER2-positive and PD-L1 positive (assessed ≤6 months prior to study entry) who have been treated with trastuzumab (±pertuzumab) and taxanes.
Ladiratuzumab vedotin combined with pembrolizumab as first-line treatment for advanced TNBC in a phase Ib/II study[10] showed an ORR of 54% among 26 evaluable patients who were not pre-selected for LIV-1 or PD-L1 expression.
The Morpheus-TNBC study is an umbrella study exploring the efficacy and safety of various combined immunotherapy regimens, currently conducting a cohort study of goserelin (SG) combined with pembrolizumab as first-line treatment for advanced TNBC. The Saci-IO TNBC study is exploring SG combined with pembrolizumab for clinical research in PD-L1 negative advanced TNBC. Additionally, SG combined with pembrolizumab is also being explored in HR-positive advanced breast cancer (NCT04448886)[6].
The above research progress indicates that ADCs and immune checkpoint inhibitors have potential synergistic activity, and multiple clinical studies are actively underway, expected to overcome resistance to current treatments and provide more therapeutic options for breast cancer patients. The early data from studies on the combination of ADCs and immune checkpoint inhibitors have not shown significant additive toxicity, including the DS8201-A-U105 study, whose safety profile is similar to previous reports of DS-8201 monotherapy, all suggesting the safety of this combined approach.
Table 1. Research Progress of ADC Combined with Immune Checkpoint Inhibitors in Breast Cancer
Conclusion
Based on previous preclinical and clinical research explorations, ADC combined with immunotherapy remains a promising treatment model, and we look forward to more data disclosures in the future, as Professor Bardia stated, to develop more rational ADC drug and immunotherapy combination schemes.
Expert Profile

Professor Liu Yunjian
Deputy Director of the Fourth Hospital of Hebei Medical University
PhD, Professor, Doctoral Supervisor
Outstanding Young and Middle-aged Expert in Hebei Province
Leader of the Surgery Discipline at Hebei Medical University
Vice Chairman of the Breast Disease Professional Committee of the Chinese Medical Education Association
Executive Vice Chairman of the Health Science Popularization Professional Committee of the Chinese Health Management Association
Member of the Breast Surgery Group of the Surgical Branch of the Chinese Medical Association
Standing Committee Member of the Tumor Plastic Surgery Professional Committee of the Chinese Anti-Cancer Association
Standing Committee Member of the Breast Cancer Professional Committee of the Chinese Anti-Cancer Association
Standing Committee Member of the Breast Cancer Expert Committee of the Chinese Society of Clinical Oncology
Standing Committee Member of the Breast Surgery Physician Committee of the Chinese Physician Association
Vice Chairman and Secretary-General of the Hebei Anti-Cancer Association
Executive Chairman of the Hebei Cancer Prevention and Treatment Alliance
References:
[1] ERIKA HAMILTON, CHARLES L. SHAPIRO, VALENTINA BONI, et al. PRIMARY ANALYSIS FROM DS8201-A-U105: A 2-PART, OPEN-LABEL, PHASE 1B TRIAL ASSESSING TRASTUZUMAB DERUXTECAN (T-DXd) WITH NIVOLUMAB IN PATIENTS WITH HER2-EXPRESSING ADVANCED BREAST CANCER. 2022 ESMO BC. 162O.
[2] Erika Hamilton, Charles L. Shapiro, Daniel Petrylak, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing advanced breast cancer: a 2-part, phase 1b, multicenter, open-label study. 2020 SABCS. PD3-07.
[3] Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-Targeting Antibody-Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol Cancer Ther. 2018 Jul;17(7):1494-1503.
[4] Saini KS, Punie K, Twelves C, et al. Antibody-drug conjugates, immune-checkpoint inhibitors, and their combination in breast cancer therapeutics. Expert Opin Biol Ther. 2021 Jul;21(7):945-962.
[5] Peter Schmid, Seock-Ah Im, Anne Armstrong, et al. BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). 2021 ASCO. 1023.
[6] Home – ClinicalTrials.gov
[7] Waks AG, Keenan T, Li T, et al. A phase Ib study of pembrolizumab (pembro) plus trastuzumab emtansine (T-DM1) for metastatic HER2+ breast cancer (MBC). J Clin Oncol. 2020;38(15_suppl):1046-1046. doi:10.1200/JCO.2020.38.15_suppl.1046
[8] Hamilton EP, Kaklamani V, Falkson C, et al. Abstract PD1-05: Atezolizumab in combination with trastuzumab emtansine or with trastuzumab and pertuzumab in patients with HER2-positive breast cancer and atezolizumab with doxorubicin and cyclophosphamide in HER2-negative breast cancer: Safety and biomarker outcomes from a multi-cohort Phase Ib study. Cancer Res. 2020;80(4 Supplement):PD1-PD1-05. doi:10.1158/1538-7445.SABCS19-PD1-05
[9] Emens LA, Esteva FJ, Beresford M, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21(10):1283-1295. doi:10.1016/S1470-2045(20)30465-4
[10] Han H (Heather), Diab S, Alemany C, et al. Abstract PD1-06: Open label phase 1b/2 study of ladiratuzumab vedotin in combination with pembrolizumab for first-line treatment of patients with unresectable locally-advanced or metastatic triple-negative breast cancer. Cancer Res. 2020;80(4Supplement):PD1-PD1-06. doi:10.1158/1538-7445.SABCS19-PD1-06

ADC Academy Online
The ADC Academy is dedicated to disseminating the history and progress of antibody-drug conjugates (ADCs), keeping up with the latest advancements in related therapeutic fields, and providing a comprehensive platform for academic exchange for professionals in the field.