
In recent years, with the increasing attention to health, environmental protection, and sustainable resource development, plant-based foods have become increasingly favored by consumers. Traditional soybean products such as tofu, bean curd, and vegetarian chicken, as well as new products containing soybean protein, have developed rapidly in the domestic market, leading to a growing demand for soybean products. The quality of soybean products (texture, water retention, flavor, etc.) largely depends on the gel properties of soybean protein[1]. Achieving high-quality, stable gel products has always been a research focus in this field[2]. Research shows that the aggregation of proteins is crucial for the formation of gel structure and quality, with large protein aggregates serving as the “precursors” for gel network formation[3]. Studies indicate that under the action of coagulants, large protein aggregates first aggregate to form the “framework” of the gel, followed by the further binding of smaller protein aggregates or soluble proteins onto the framework, resulting in the final gel structure[4]. The formation of protein gels can be considered a result of the aggregation of denatured proteins in a specific order, from protein molecules to primary particles, microstructures, and gel networks. The transition from micro to macro structures collectively determines different colloidal structures, with the closest structural hierarchy to the macro world being the main factor governing colloidal performance[5]. Achieving control over the gelation process is an effective means to enhance the properties of protein gels. By adjusting the aggregation or cross-linking reactions between protein molecules, food gels with desired properties and microstructures can be obtained[6]. Currently, studies have utilized multiple emulsions (W/OW) or the addition of polysaccharides to control the release of coagulants for sustained release effects, thus improving the gelation ability of proteins[7-8], but these methods have certain limitations, such as complex operations and the introduction of external additives, which restrict their application and development in food processing.
Metal salt ions (hereinafter referred to as salt ions) induce protein gelation, which is a very important method in the processing of traditional soybean products. Commonly used salt coagulants mainly include some divalent metal salt ions, including calcium and magnesium salts[8-9]. It has been reported that the concentration of salt ions has a significant impact on the formation of protein gels. At low ionic strengths, proteins tend to form fine chain networks, while at high ionic strengths, they form particle networks[10]. In addition, different salts have different coagulation abilities for proteins. Compared to MgCl2 and CaCl2, the gel structure induced by CaSO4 is more uniform and delicate[11]. Mohammadian et al.[12] studied the effects of CaCl2, MnCl2, and ZnCl2 on gel structure and found that gels induced by Zn2+ are harder and have denser microstructures than those induced by other salt ions. Therefore, it can be seen that there are significant differences in the aggregation behavior of proteins under different salt ions, and the process of protein aggregation and gelation can be regulated by changing the addition method of salt ions (type, concentration, etc.), thus achieving effective improvement of gel structure and quality. Currently, there are few studies on the interactions between different salt ions and protein molecules and the gelation performance of protein aggregates induced by different salts.
This study aims to utilize the salt ion type and concentration-dependent characteristics of protein aggregation behavior, using soy protein isolate (SPI) as the research object, to employ a new two-step method for preparing salt-induced SPI gel. In the first step, at the same total salt ion concentration level (35 mmol/L), different salt ions (CaSO4, MgSO4, or ZnSO4) are used to induce SPI aggregation (pre-aggregation) at low concentrations (0-15 mmol/L) to prepare protein particles (gel precursors) with different structures and properties, comparing the differences in the effects of different salt ions on SPI structure and aggregate formation; in the second step, a high concentration coagulant (20-35 mmol/L, CaSO4) is added to induce the gelation of protein particles, studying the gelation performance of SPI aggregates with different structures and properties, and exploring the effect of salt ion pre-aggregation treatment on SPI gel properties. It is hoped that the results of this study can provide a theoretical basis and new ideas for the processing and quality improvement of soybean products and even plant protein gel products.
1.1 Materials and Reagents
Defatted soybean meal, Shandong Yuwang Ecological Food Industry Co., Ltd.; all chemical reagents used in the experiment are of analytical grade.
1.2 Instruments and Equipment
GL-10MD large-capacity high-speed refrigerated centrifuge, Hunan Xiangyi Centrifuge Instrument Co., Ltd.; FD-1A-50 freeze dryer, Shanghai Hefan Company; MS3000 laser particle size analyzer, Malvern Instruments, UK; MCR302 rotary rheometer, Anton Paar GmbH, Austria; J-1500 circular dichroism spectrometer, JASCO Corporation, Japan; FluoroMax-4 high-sensitivity fluorescence spectrometer, Horiba Ltd., France; Nano ITC SV isothermal titration calorimeter, TA Instruments, USA; TA-XT Plus texture analyzer, Stable Micro Systems, UK.
1.3 Experimental Methods
1.3.1 Preparation of SPI
Defatted soybean meal was mixed with deionized water at a mass ratio of 1:10, and the pH was adjusted to 8.0 using 2 mol/L NaOH. The mixture was stirred at room temperature for 2 hours, centrifuged (7000 r/min, 4 ℃) for 20 minutes, and the supernatant was adjusted to pH 4.5 using 2 mol/L HCl, followed by centrifugation (4000 r/min) for 10 minutes to obtain the precipitate. The precipitate was dissolved in deionized water at a mass ratio of 1:2 and adjusted to pH 7.0 using 2 mol/L NaOH until completely dissolved. The protein solution was freeze-dried to obtain SPI powder, which was stored at -80 ℃. The protein mass fraction was 92 g/100g, fat mass fraction was 0.5 g/100g, carbohydrate mass fraction was 1.0 g/100g, moisture mass fraction was 5.7 g/100g, and ash mass fraction was 2.8 g/100g.
1.3.2 Preparation of SPI Solution
SPI powder was dissolved in deionized water and stirred at room temperature for over 2 hours until fully dissolved. The solution was centrifuged at 7000 r/min for 10 minutes to remove insoluble substances. The pH of the supernatant was adjusted to 7.0 using 2 mol/L NaOH or HCl, and the protein concentration was adjusted to 60 mg/mL. The solution was then heated in a 95 ℃ water bath for 30 minutes and cooled in cold water to room temperature for later use.
1.3.3 Thermodynamic Measurement of Interactions between Different Salt Ions and Heat-Denatured SPI
The interactions between different salt ions and SPI were tested using an isothermal titration calorimeter. The SPI solution concentration from section 1.3.2 was diluted to 2 mg/mL, and solutions of 10.0 mmol/L CaSO4, MgSO4, and ZnSO4 were prepared (the selection of salt solution concentration was based on the solubility of CaSO4 to ensure complete dissolution). Before starting the experiment, the instrument, sample injection needle, and titration needle were cleaned, and all solutions were degassed for 10 minutes before sample loading. Experimental parameters were set as follows: temperature at 25 ℃, stirring speed at 250 r/min, each titration volume at 5 μL, with a 300 s interval between two titrations, for a total of 18 titrations. The blank experiment was the titration of salt ion solution against primary water, while the interaction experiment was the titration of salt ion solution against SPI solution.
1.3.4 Structural Measurement of SPI Pre-Aggregates Induced by Different Salt Ions
1.3.4.1 Measurement of SPI Secondary Structure
Solutions of CaSO4, MgSO4, and ZnSO4 were added to the heat-denatured SPI solution to achieve a salt ion concentration of 10.0 mmol/L, and stirred for 1 hour. The treated SPI aggregate solution was then diluted to a concentration of 0.1 mg/mL using 0.01 mol/L (pH 7.0) phosphate buffer solution. The secondary structure of the pre-treated SPI was measured using circular dichroism spectroscopy under conditions: sample cell light path 0.1 cm, ambient temperature 25 ℃, sensitivity 20 mdeg, scanning speed 100 nm/min, with three scans per sample to generate data, and a scanning range of 190-250 nm. Each sample was measured three times for repetition.
1.3.4.2 Measurement of SPI Intrinsic Fluorescence Spectrum
The intrinsic fluorescence spectrum of SPI was measured using a high-sensitivity fluorescence spectrometer. Solutions of CaSO4, MgSO4, and ZnSO4 were added to the heat-denatured SPI solution to achieve a salt ion concentration of 10.0 mmol/L, and stirred for 1 hour. The solution was then diluted to 0.4 mg/mL using 0.01 mol/L (pH 7.0) phosphate buffer solution. Following the method of Wang Zhongjiang et al.[13] with slight modifications, the tryptophan fluorescence groups within the protein molecules were used as probes. To reduce the impact of tyrosine on the fluorescence spectrum, the excitation wavelength was set to 290 nm, with the emission spectrum scanning range of 300-450 nm and a slit width of 5 nm.
1.3.5 Preparation of SPI Gel
1.3.5.1 Preparation of SPI Pre-Aggregated Particles
Different concentrations (2.5, 5.0, 7.5, 10.0, 15.0 mmol/L) of different salt solutions (CaSO4, MgSO4, ZnSO4) were added to the heat-denatured SPI solution (concentration 60 mg/mL), stirred at 400 r/min for 1 hour to allow sufficient reaction, resulting in SPI pre-aggregated particles.
1.3.5.2 Preparation of SPI Gel
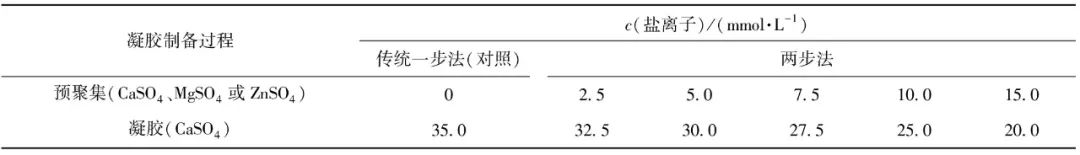
To the solution containing different SPI pre-aggregated particles, CaSO4 (coagulant, suitable for preparing filled gels) was added until the final concentration of metal salt ions reached 35 mmol/L, and gelation was induced in a 80 ℃ water bath for 30 minutes. The mixture was then cooled to room temperature and stored in a 4 ℃ refrigerator overnight for later use. The proportions of salt ions added during the two-step preparation of SPI gel are shown in Table 1. The traditional one-step method, in which 35 mmol/L CaSO4 is added at once to induce SPI gel, serves as a control.
Table 1 Proportions of Salt Ions Added during the Two-Step Preparation of SPI Gel
1.3.6 Measurement of Particle Size of SPI Pre-Aggregates
The particle size of SPI pre-aggregates was measured using a laser particle size analyzer. The SPI aggregate solution was slowly added to the measurement pool until the refractive index reached about 7%. The continuous phase refractive index was taken as 1.45 (protein), and the dispersive phase refractive index was 1.33 (water). The particle size of SPI pre-aggregates was expressed as the volume-weighted average particle size (D4,3), with multiple measurements taken to obtain an average value.
1.3.7 Measurement of Apparent Viscosity of SPI Pre-Aggregate Solution
The apparent viscosity of the SPI solution after pre-aggregation treatment was measured using a rotary rheometer, employing the cone-plate CP25 under rotational mode, with shear rates ranging from 0.1 to 100.0 rad/s. The sample was equilibrated at 25 ℃ for 2 minutes before measurement commenced. The flow curve was generated as a function of shear rate, and the Ostwald de Waele rheological model was used for fitting, as shown in equation (1).
τ=Kγn.
(1)
In equation (1), τ is the shear stress in Pa; γ is the shear rate in s-1, K (Pa·s) is the viscosity coefficient, and n is the flow behavior index.
1.3.8 Measurement of Viscoelasticity of SPI Gel
The viscoelasticity of SPI gel was measured using a rheometer, with the rotor chosen as PP50 and a gap value of 1 mm. As described in 1.3.5, after adding the coagulant, the mixture was homogenized and immediately loaded onto the rheological testing platform. The program was initiated, and edge scraping was performed as per system instructions. To prevent moisture loss due to evaporation at high temperatures, a layer of silicone oil was applied around the rotor before conducting the temperature scanning test. Experimental parameters were set as follows: strain at 1% (within the linear range), frequency at 1 Hz. The temperature program was set to first increase from 25 ℃ to 80 ℃, maintaining at 80 ℃ for 30 minutes, then cooling down to 25 ℃. Frequency scanning was conducted on the formed SPI gel, with experimental parameters set as: strain at 1%, angular frequency (ω) ranging from 1.0 to 100.0 rad/s, recording the variations of elastic modulus (storage modulus) G′ and viscous modulus (loss modulus) G″ with angular frequency. The relationship between angular frequency and SPI gel modulus was analyzed using a power law model, with the fitting formula shown in equation (2).
G=Kωn.
(2)
In equation (2), K is the power law constant, and n indicates the degree of dependence of the modulus on angular frequency.
1.3.9 Measurement of SPI Gel Hardness
The hardness of SPI gel was measured using a texture analyzer. The SPI gel prepared in 1.3.5 was taken out and allowed to return to room temperature. A P/0.5 probe was used, and the sample was placed on the measuring platform for determination. Experimental parameters were set as follows: pre-measurement and post-measurement speed at 5 mm/s, measurement speed at 2 mm/s, penetration depth at 70% of the gel height, and trigger force at 0.05 N. The peak pressure during the penetration of the sample was defined as the hardness of the gel. At least three tests were conducted to obtain an average value.
1.4 Data Processing and Analysis
All data were measured at least three times, with at least three parallels for each measurement, and results are expressed as mean ± standard deviation. SPSS Statistics 26 software was used for variance and correlation analysis, and the LSD method was applied for significant analysis of the experimental data (P<0.05).
2.1 Thermodynamic Analysis of Interactions between Different Salt Ions and SPI
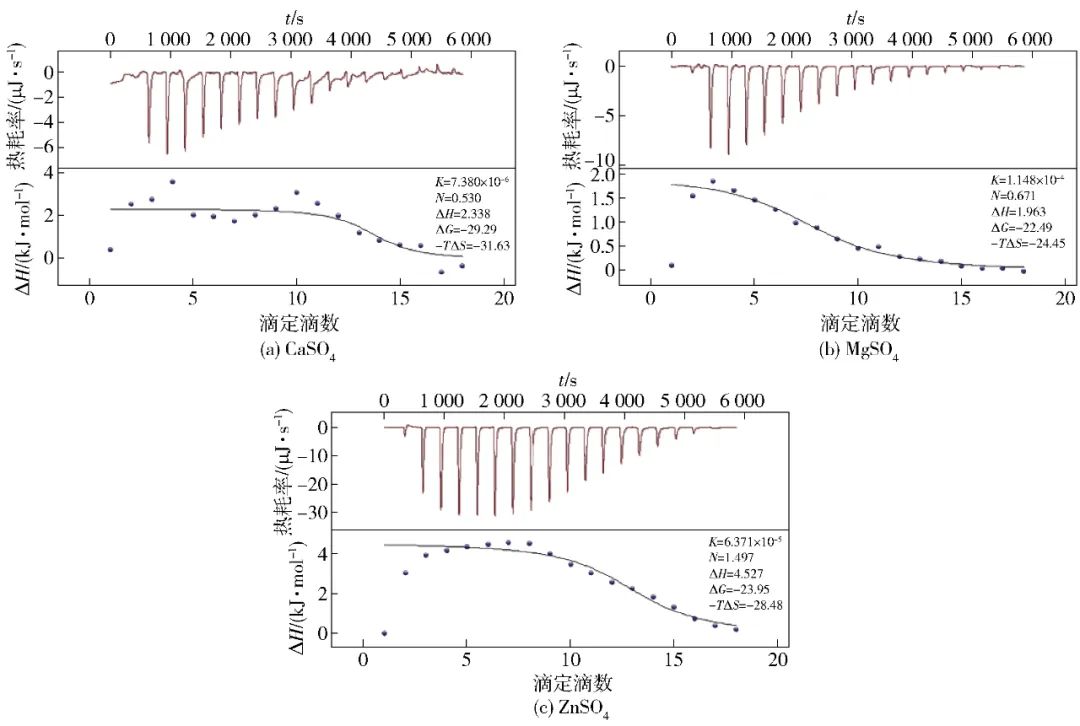
Figure 1 ITC Analysis Results of Interactions between Different Salt Ions and SPI
To analyze the interactions between different salt ions and protein molecules, the heat changes generated during the binding of salt ions to proteins were monitored using isothermal titration calorimetry (ITC) (Figure 1). Thermodynamic parameters during the interaction were obtained through fitting based on the thermodynamic equations[14-15]. From Figure 1, it can be seen that the area of each peak corresponds to the heat generated during the titration of one drop of salt ion solution, and as the amount of titrated salt ions increases, the peak height gradually decreases, indicating a reduction in the heat generated. When the heat generated approaches zero, it indicates that the titration endpoint has been reached, and the SPI molecules are completely bound. Further titration will only produce positive dilution heat, and the peak height tends to stabilize. From the heat change, it is evident that the heat change caused by titration with ZnSO4 is much greater than that of CaSO4 and MgSO4, indicating a more intense aggregation reaction of protein molecules induced by Zn2+. According to the thermodynamic parameters obtained from fitting, the interaction between different salt ions and SPI is spontaneous (ΔG<0) and endothermic (ΔH>0), consistent with the results of Canababy et al.[16]. Furthermore, Ross et al.[17] and Shi Huigang et al.[18] summarized the thermodynamic laws of the interactions between biomacromolecules and small molecules, indicating that when ΔH<0, the interaction is primarily electrostatic, van der Waals forces, or hydrogen bonding, and when ΔS>0, it is electrostatic interaction, while ΔS<0 indicates van der Waals forces or hydrogen bonding; when ΔH>0 and ΔS>0, it indicates hydrophobic interactions. Throughout the entire reaction process of this experiment, ΔH>0 and ΔS>0, indicating that the interaction between salt ions and SPI molecules is driven by entropy changes, and the addition of salt ions primarily manifests as hydrophobic interactions among protein molecules. Additionally, the fitted enthalpy changes (ΔH) from the interactions between different salt ions and SPI reveal that the enthalpy changes caused by CaSO4 and MgSO4 are similar, while the titration with ZnSO4 induces relatively larger enthalpy changes, further demonstrating the differences in interactions between different salt ions and SPI.
2.2 Effects of Different Salt Ions on the Spatial Conformation of SPI
2.2.1 Effects of Different Salt Ion Pre-Aggregation Treatments on SPI Secondary Structure
To further analyze the effects of different salt ion pre-aggregation treatments on the aggregate structure of SPI, the spatial conformations of pre-aggregated and non-pre-aggregated SPI were measured, with experimental results shown in Figure 2. From Figure 2, it can be seen that all samples exhibited a positive peak around 190-195 nm, indicating the presence of β-sheet structures in SPI; a negative peak was observed near 210 nm, characteristic of the typical α-helix structure in SPI[19]. Moreover, after different salt ion pre-aggregation treatments, the circular dichroism spectra of SPI samples shifted to a redder wavelength to varying degrees. The negative peak of the heat-denatured SPI shifted from 210 nm to 214, 212, and 216 nm after the addition of CaSO4, MgSO4, and ZnSO4, respectively, indicating an increase in the formation of α-helix structures. The secondary structure content of SPI was obtained by fitting the circular dichroism results, as shown in Table 2. Table 2 shows that the α-helix and β-sheet structures are the primary secondary structures of SPI. Compared to the original SPI, the α-helix structure content in the heat-treated SPI secondary structure significantly decreased, while the random coil content increased. This is due to the denaturation of SPI after heat treatment, causing the protein molecular structure to unfold, breaking intermolecular hydrogen bonds, as the α-helix structure is maintained by hydrogen bonds between the protein’s carbonyl and amino groups[20], which leads to a decrease in α-helix structure content and a transition from α-helix structure to random coils. However, upon further addition of different salt ions, the SPI samples exhibited an increase in α-helix structure content and a decrease in β-sheet structure content. Literature has reported that the content of protein α-helix structure is inversely proportional to its surface hydrophobicity[21], thus this phenomenon may be attributed to the electrostatic shielding effect of salt ions and the formation of salt bridges between proteins and metal salt ions, causing protein aggregation and leading to the re-embedding of exposed hydrophobic groups into the interior, thereby reducing the protein’s surface hydrophobicity and increasing the α-helix structure content while decreasing the β-sheet structure content. Additionally, some studies suggest that the increase in α-helix structure content is due to the aggregation of proteins through intermolecular interactions after the addition of salt solutions, leading to the re-establishment of hydrogen bonds between peptide chains of protein molecules[22-23]; however, other studies have indicated that salt ions can affect the stability of hydrogen bonds between peptide chains of protein molecules by altering the electrostatic interactions between them, thereby reducing the α-helix structure content in SPI[24].
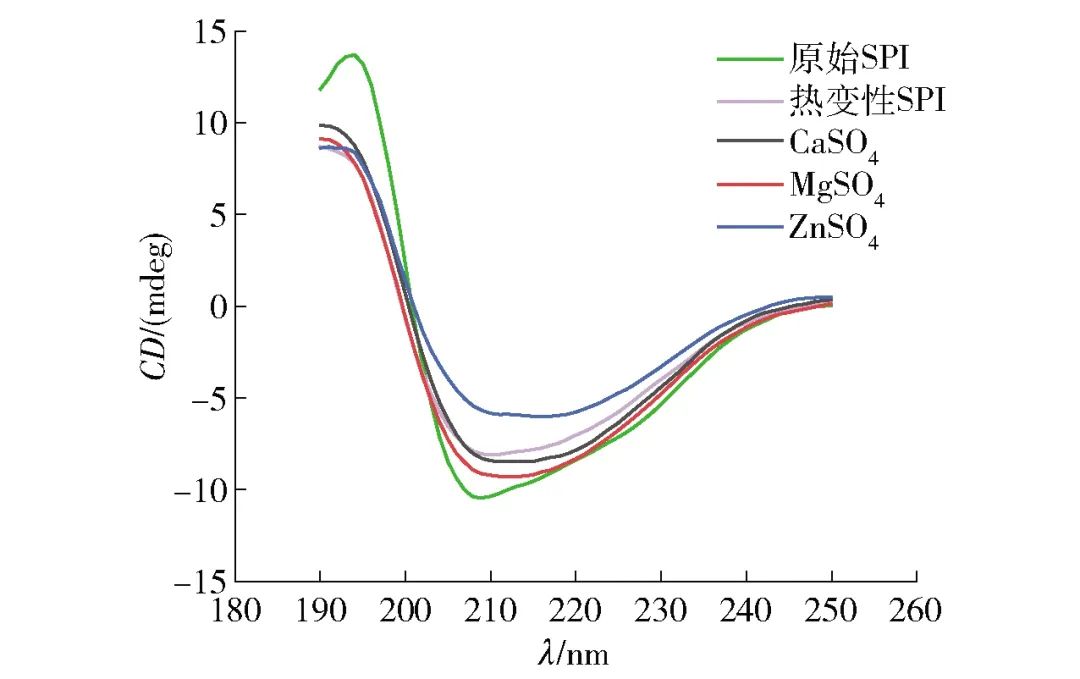
Figure 2 Circular Dichroism of SPI under Different Salt Ion Pre-Aggregation Treatments
Table 2 Secondary Structure Content of SPI under Different Salt Ion Pre-Aggregation Treatments
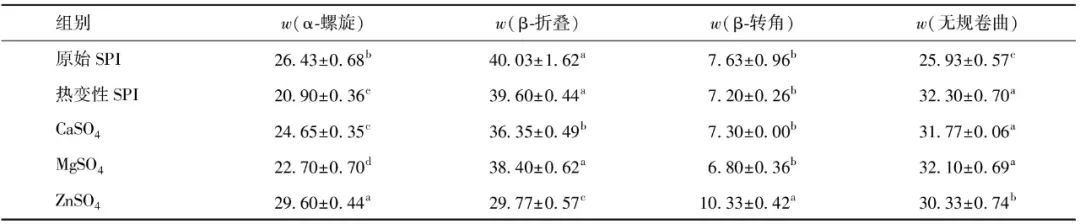
Different letters in the same column indicate significant inter-group differences (P<0.05).
2.2.2 Effects of Different Salt Ion Pre-Aggregation Treatments on SPI Fluorescence Spectrum
Figure 3 shows the fluorescence spectrum of SPI after different salt ion pre-aggregation treatments. The fluorescence spectrum of SPI samples excited at 290 nm mainly comes from tryptophan emission, reflecting changes in the microenvironment of tryptophan residues[25]. Tryptophan residues are hydrophobic residues, and their fluorescence peaks generally appear in the range of 325-350 nm[13]. As seen in Figure 3, the fluorescence spectra of all SPI samples are nearly identical, while the fluorescence intensity of the heat-treated SPI samples increased, and the fluorescence intensity of SPI samples with different salt ions decreased. This is due to the denaturation of the SPI upon heating, which causes the molecular structure to unfold and exposes the hydrophobic groups within the protein, placing the tryptophan residues in a polar environment outside the protein. The decrease in fluorescence intensity after the addition of salt ions can be attributed to the interaction between divalent metal ions and the polar surfaces of proteins, leading to further protein aggregation and causing the exposed tryptophan residues to be re-embedded into the interior of protein aggregates, resulting in fluorescence quenching[26], which is consistent with the results from circular dichroism.
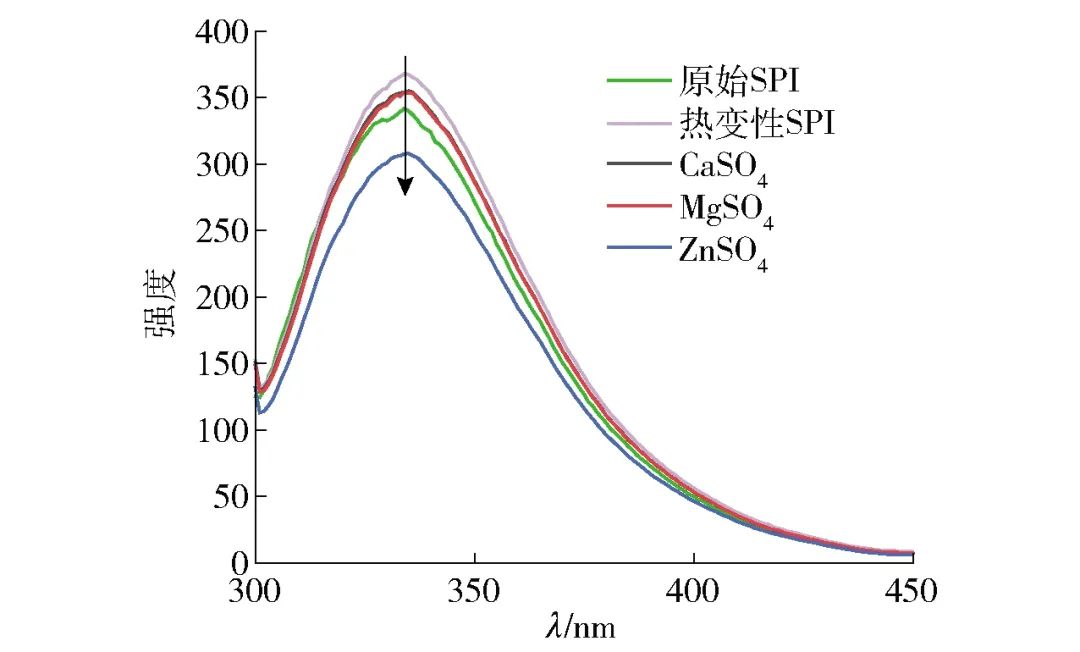
Figure 3 Fluorescence Spectrum of SPI under Different Salt Ion Pre-Aggregation Treatments
2.3 Effects of Different Salt Ion Pre-Aggregation Treatments on SPI Particle Size
The size of protein aggregates is closely related to gel structure and performance. The differences in protein aggregation behavior induced by different salt ions were significant, and the particle sizes of SPI aggregates formed under different salt ions were measured using dynamic light scattering technology, with the volume-weighted average particle size D4,3 shown in Table 3. From Table 3, it can be seen that as the concentration of salt ions increases, the D4,3 of SPI aggregates gradually increases. For different salt ions, at low concentrations (2.5-7.5 mmol/L), the particle size induced by CaSO4 is significantly larger than that induced by MgSO4; however, at high concentrations (10-15 mmol/L), there is no significant difference in D4,3 between the two salt ions (P<0.05). In comparison, under the influence of ZnSO4, proteins exhibited a higher degree of aggregation, resulting in larger aggregate particle sizes. The differences in D4,3 of SPI aggregates after the addition of different salt ions may be attributed to differences in the aggregation rates and abilities of the salt ions.
Table 3 Particle Sizes of SPI Aggregates Induced by Different Salt Ions
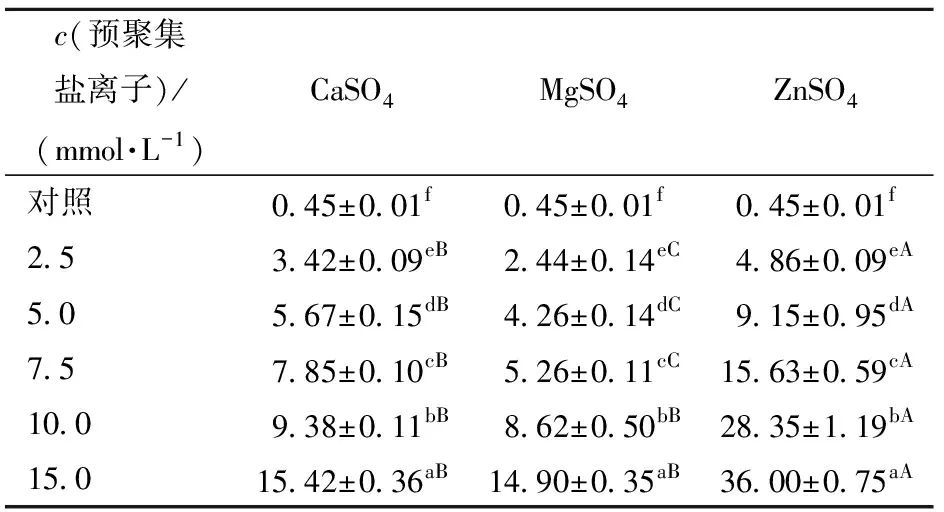
Different lowercase letters in the same column indicate significant differences between samples treated with the same salt ion at different salt concentrations (P<0.05); different uppercase letters in the same row indicate significant differences between samples treated with different salt ions at the same concentration (P<0.05).
This is due to the different aggregation rates and abilities of salt ions. The ITC results indicate that the reaction intensity between ZnSO4 and protein molecules is much higher than that of CaSO4 and MgSO4, leading to rapid aggregation of protein molecules that do not have time to rearrange, forming larger and rougher SPI aggregate particles; while salt ions with slower aggregation rates will gradually induce protein molecules to aggregate, forming uniform and smaller SPI aggregate particles.
2.4 Effects of Different Salt Ion Pre-Aggregation Treatments on SPI Solution Apparent Viscosity
Figure 4 shows the changes in the apparent viscosity of SPI aggregate solutions induced by three salt ions under different concentration conditions as a function of shear rate. Figure 4 indicates that with the increase in shear rate, the viscosity of all SPI aggregate solutions gradually decreases, exhibiting a shear-thinning phenomenon, indicating that SPI aggregate solutions are typical non-Newtonian fluids. Furthermore, the increase in salt ion concentration can enhance the interactions between proteins, thus, when the solution is stable, the higher the concentration of salt ions, the higher the apparent viscosity of the SPI aggregate solution. The flow behavior indices of SPI solutions after different salt ion pre-aggregation treatments are shown in Table 4. From Table 4, it can be seen that the sample pre-aggregated with 15.0 mmol/L ZnSO4 exhibited poor fitting of the flow curve due to its high viscosity, while the flow curves of the other samples demonstrated good fitting (R2>0.99). When the flow behavior index of a sample is 1, it is considered a Newtonian fluid; if the flow behavior index approaches 1, it indicates better fluidity; conversely, it indicates rigidity[27-29]. The results of this study indicate that the flow behavior index of samples gradually decreases with the increase in the concentration of pre-aggregated salt ions, suggesting that the aggregation degree of SPI increases with the concentration of salt ions. Additionally, at the same concentration, the flow behavior indices of SPI solutions pre-aggregated with ZnSO4 are lower than those of CaSO4 and MgSO4, indicating a stronger aggregation ability of ZnSO4. This may be related to the ionic radius of different salt ions; research by Mohammadian et al.[12] indicated that the ionic radius plays an important role in the interactions between protein molecules. Compared to other salt ions, zinc ions have a smaller radius and higher charge density, thus exhibiting stronger cross-linking ability with proteins, although the specific mechanism requires further investigation.
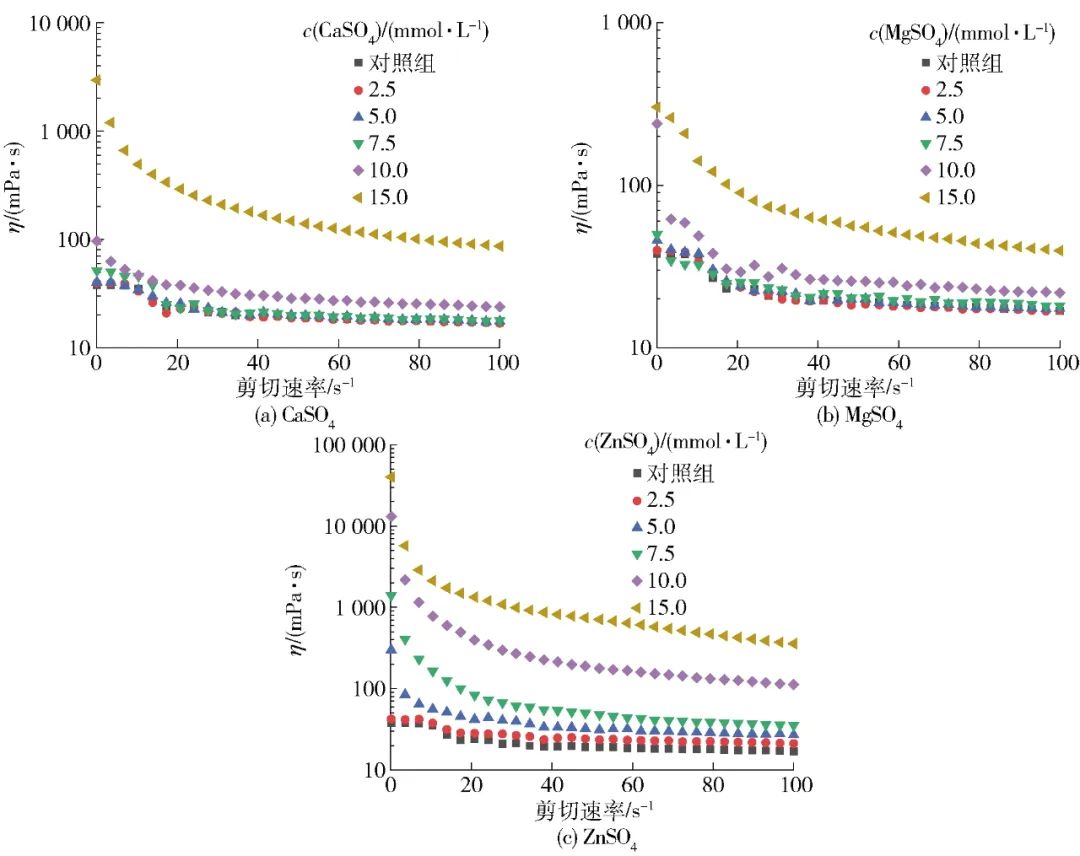
Figure 4 Changes in Apparent Viscosity of SPI Aggregate Solutions with Shear Rate
Table 4 Flow Behavior Indices of SPI Solutions after Different Salt Ion Pre-Aggregation Treatments
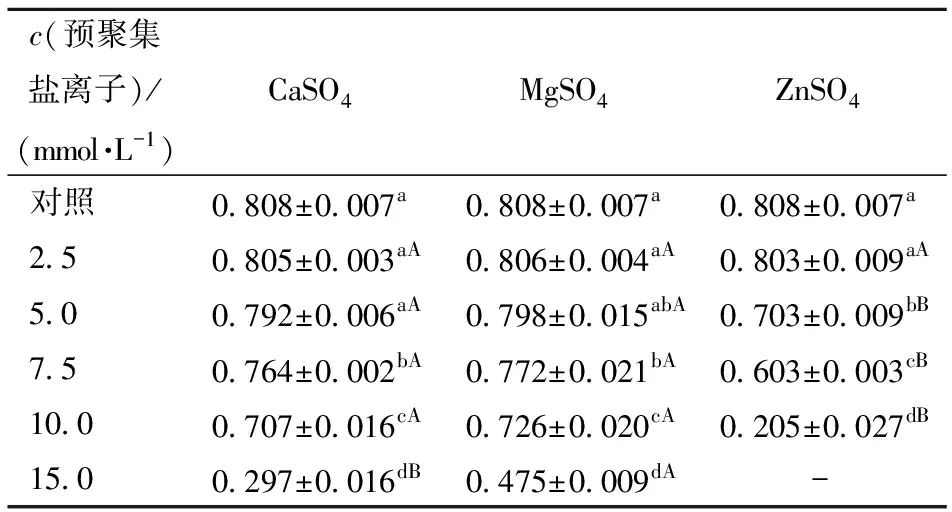
Different lowercase letters in the same column indicate significant differences between samples treated with the same salt ion at different salt concentrations; different uppercase letters in the same row indicate significant differences between samples treated with different salt ions at the same concentration (P<0.05). “-” indicates that the sample’s flow curve fitting is poor, and thus the data is not highly referential.
2.5 Effects of Different Salt Ion Pre-Aggregation Treatments on SPI Gel Viscoelasticity
To investigate the effects of different salt ion pre-aggregation treatments on SPI gel viscoelasticity, frequency scanning experiments were conducted on SPI gels, with results shown in Figure 5. The elastic modulus (G′) and viscous modulus (G″) respectively describe the solid (elastic) and liquid (viscous) characteristics of SPI gel samples[30]. Figure 5 shows that all SPI gel samples exhibited obvious gel characteristics, with G′>G″, and both G′ and G″ of the gel gradually increase with the increase in angular frequency ω across the entire frequency range. The dynamic viscoelastic behavior of all SPI gel samples was further studied using a power law model to fit the relationship between G′ and angular frequency (ω). The K value indicates the power law constant of the fitting model and can be used to assess the elasticity of the gel, while the exponent n represents the degree of dependence of G′ on ω. Additionally, the n value can characterize the nature of the forces acting on the gel structure. When n>0, it indicates a physical gel primarily formed by weak non-covalent bonds; when n=0, it indicates a chemical gel primarily composed of stronger covalent bonds, generally showing no dependency of gel modulus on frequency[32]. The fitting results of G′ and ω are shown in Table 5. Most SPI gel samples have an n value around 0.1, indicating that G′ exhibits weak frequency dependence, and the gel induced by salt ions is a physical weak gel formed mainly by ionic bonds, hydrophobic interactions, and hydrogen bonds. Within a certain range, as the concentration of salt ions increases, the K value of SPI gel significantly increases, indicating that pre-aggregation treatment can greatly improve the gel’s viscoelasticity. According to Table 5, the gels pre-aggregated with 10.0 mmol/L CaSO4, 10.0 mmol/L MgSO4, and 5.0 mmol/L ZnSO4 exhibit the best elastic properties, with K values increasing by 49.5%, 30.0%, and 59.9% respectively compared to the control sample. However, excessive pre-aggregation treatment can lead to a significant decrease in gel viscoelasticity, which may be attributed to the different gelation performance of SPI aggregate particles formed during the pre-aggregation process. Under lower salt ion concentration conditions (CaSO4 and MgSO4 concentrations below 10 mmol/L, ZnSO4 concentration below 5 mmol/L), the aggregation reaction induced by salt ions is relatively mild, resulting in larger, compact aggregate particles that help form SPI gels with rough chains and uniformly dense networks, thereby enhancing gel structure and performance. When the salt concentration further increases, the reaction rate accelerates rapidly, leading to random aggregation of protein molecules, easily forming overly large and rough aggregates, which results in an uneven gel network and lower viscoelasticity[32-34].
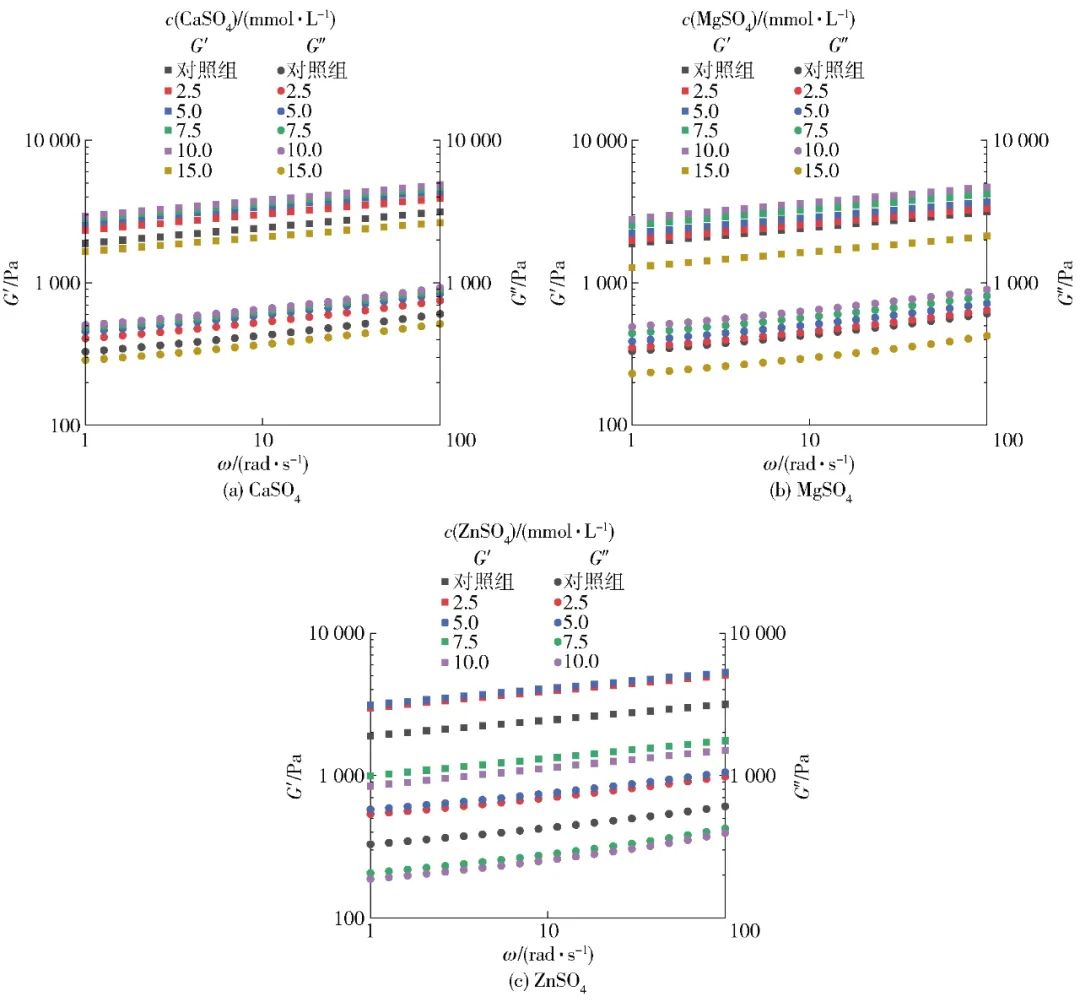
Figure 5 Elastic Modulus and Viscous Modulus of SPI Gel under Different Salt Ion Pre-Aggregation Treatments
Table 5 Fitting K and n Values of Power Law Model
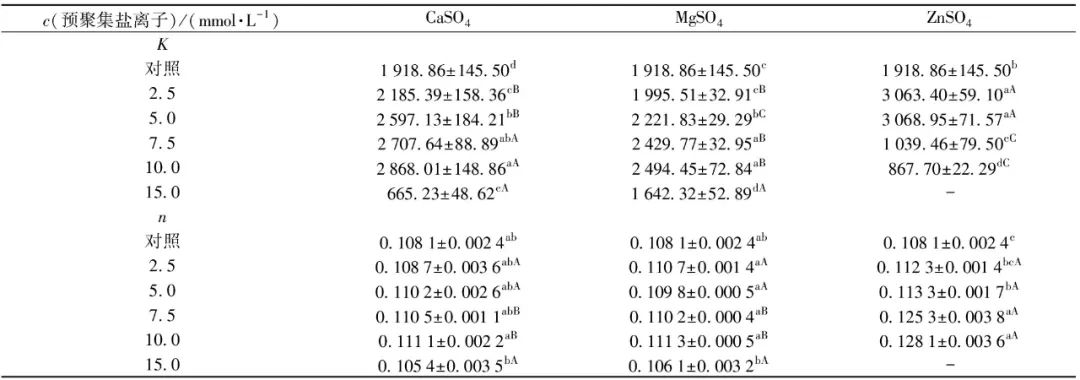
Different lowercase letters in the same column indicate significant differences between samples treated with the same salt ion at different salt concentrations; different uppercase letters in the same row indicate significant differences between samples treated with different salt ions at the same concentration (P<0.05); “-” indicates that the sample cannot form a gel structure.
2.6 Effects of Different Salt Ion Pre-Aggregation Treatments on SPI Gel Hardness
The effects of pre-aggregation treatment with salt ions on SPI gel hardness are shown in Figure 6. From Figure 6, it can be seen that the pre-aggregation treatment with salt ions significantly improved the hardness of SPI gel within a certain concentration range. The SPI gels pre-aggregated with 10 mmol/L CaSO4, 10 mmol/L MgSO4, and 2.5 mmol/L ZnSO4 achieved maximum hardness of 2.19, 2.16, and 2.12 N, respectively, while the hardness of the gel without pre-aggregation treatment was 1.75 N. However, when the concentration of salt ions further increased, the hardness of SPI gel decreased. On the other hand, when adding the same concentration of salt ions, the hardness of gels pre-aggregated with CaSO4 and MgSO4 were similar and exhibited consistent trends; the hardness of SPI gel pre-aggregated with ZnSO4 showed significant differences compared to the other two salt ions (P<0.05). Particularly, when the concentration of ZnSO4 increased to 7.5 mmol/L, the hardness of the gel significantly decreased, and the SPI treated with 15 mmol/L ZnSO4 was unable to form a good gel body. The results of this study indicate that appropriate pre-aggregation treatment can promote SPI to form stronger gels, while excessive treatment leads to poorer SPI gel properties, exhibiting lower gel hardness, which is almost consistent with the rheological data.
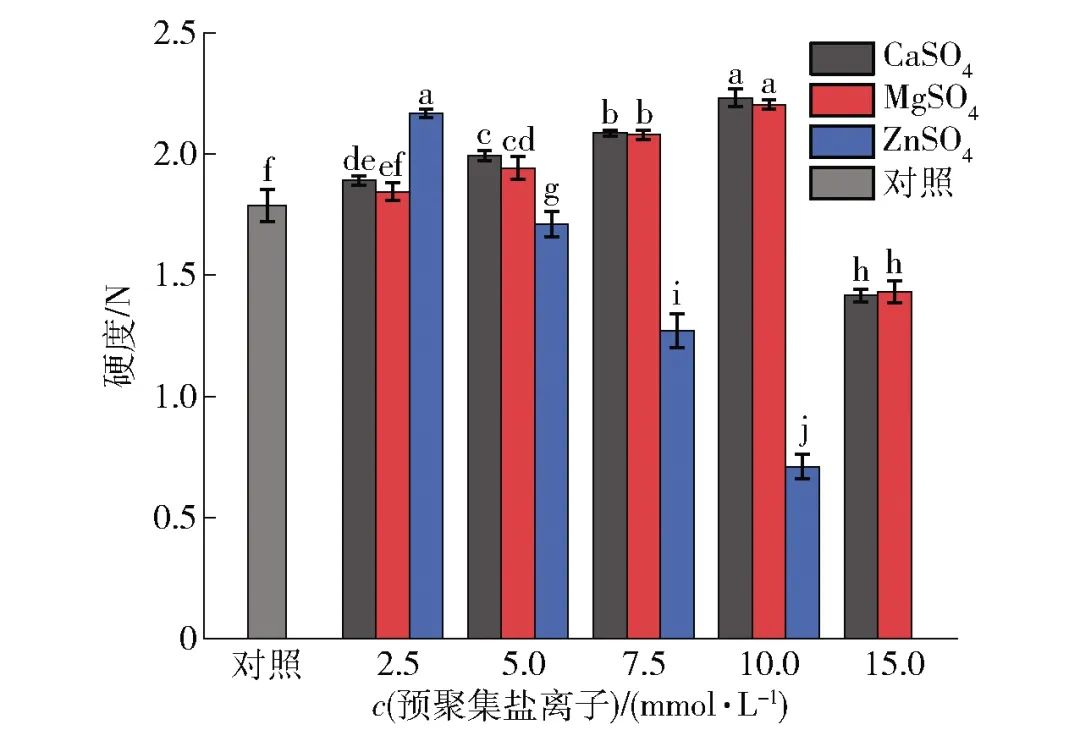
Different lowercase letters indicate significant differences between samples (P<0.05). Figure 6 Changes in Hardness of SPI Gel under Different Salt Ion Pre-Aggregation Treatments
This study utilized ITC, circular dichroism, fluorescence spectroscopy, and other methods to investigate the differences in interactions between different metal salt ions and SPI molecules and their effects on protein structure and aggregation behavior. Furthermore, by examining the changes in rheological and texture properties of SPI gels, the gelation behavior of protein aggregates induced by different salt ions was explored. The thermodynamic research results indicate significant differences in the binding of different salt ions to SPI molecules, leading to varying degrees of changes in protein structure, which in turn affects the properties of protein aggregates, such as particle size and apparent viscosity. The results from protein gel studies show that compared to the traditional one-step addition of coagulant for gelation (without pre-aggregation treatment), pre-aggregation treatment with low concentrations of salt ions can promote the formation of stronger SPI gels, significantly enhancing the gel modulus and hardness. However, excessive pre-aggregation can lead to a decline in gel properties. It is hoped that the results of this study can provide a new approach and method for the texture improvement of SPI and even plant protein gel products, offering theoretical references for the further application of SPI in gel food processing.
Citation format: Wang Xufeng, Yu Mengqin, Wang Zhenzhong, et al. Study on effect of pre-aggregation with different salt ions on gel properties of soy protein isolate based on two-step gelation method[J]. Journal of Food Science and Technology, 2022,40(1):65-75.
WANG Xufeng, YU Mengqin, WANG Zhenzhong, et al. Study on effect of pre-aggregation with different salt ions on gel properties of soy protein isolate based on two-step gelation method[J]. Journal of Food Science and Technology, 2022,40(1):65-75.

Funding Project: National Natural Science Foundation of China (32101986); Fujian Provincial Natural Science Foundation (2020J05128).