1980-Journal of the Chemical Society, Chemical Communications – Photocatalytic Complete Water Splitting Using NiO-SrTiO3: Discovery of H2 Reoxidation to Water – Kazunari Domen – University of Tokyo【Citation】Kazunari Domen, Shuichi Naito, Mitsuyuki Soma, et al.Photocatalytic Decomposition of Water Vapour on an NiO-SrTiO3 Catalyst[J].Journal of the Chemical Society, Chemical Communications, 1980, 12:543-544.【Source】
https://pubs.rsc.org/en/content/articlelanding/1980/c3/c39800000543
Kazunari Domen conducted experiments using a conventional closed gas circulation system with a 350 mL flat-bottom quartz reactor (bottom area 15 cm2). SrTiO3 powder (98.5% purity) and Ni(NO3)2 aqueous solution were injected into the flat-bottom quartz reactor, which was synthesized by calcining in air to produce NiO (1.7 wt%)-SrTiO3 photocatalyst dispersed on the bottom plane of the quartz reactor. Room temperature water vapor at 20 mmHg (0.0263 atm) was introduced into the closed gas circulation system of the quartz reactor coated with NiO (1.7 wt%)-SrTiO3 photocatalyst, and irradiated with a 450 W high-pressure mercury lamp with a water cooling system, maintaining a stable reaction temperature of 35℃. This achieved photocatalytic complete water splitting producing H2 (4.4 × 10-3 mL/h) and O2 (2.2 × 10-3 mL/h), with a molar ratio of H2: O2 = 2:1 (mol:mol).
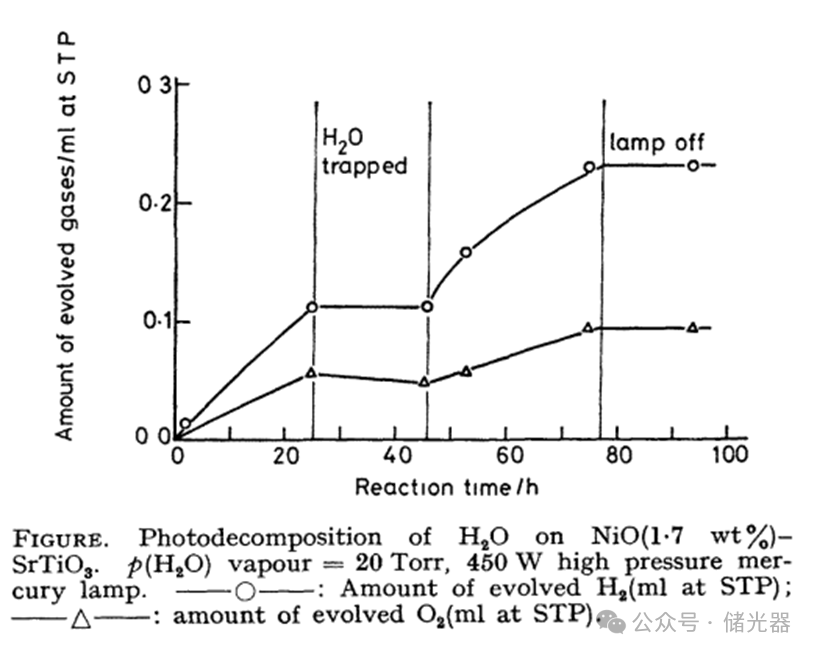
After capturing water vapor with CO2 – methanol, the photocatalytic generation of H2 and O2 immediately stopped; continuing to input water vapor resumed the photocatalytic generation of H2 and O2, which stopped immediately after turning off the light; confirming that H2 and O2 originated from the decomposition of water vapor.
Adjusting the water vapor pressure below 10 mmHg and above 15 mmHg did not change the photocatalytic hydrogen production rate, confirming that the photocatalytic hydrogen production rate is independent of water vapor pressure.
Kazunari Domen’s insight lies in his consideration of the reverse reaction of H2 and O2 generating H2O. As shown, for the NiO (1.7 wt%)-SrTiO3 photocatalyst, when water is in trace amounts, the molar amounts of H2 and O2 remain unchanged, maintaining a molar ratio of 2:1. To further determine the mechanism of NiO (1.7 wt%)-SrTiO3 photocatalytic water splitting, separate tests of NiO and SrTiO3 confirmed that they cannot produce hydrogen under the same conditions. Meanwhile, Pt-SrTiO3 and CoO-SrTiO3 can photocatalytically produce hydrogen. However, when water vapor is scarce, the H2 produced by Pt-SrTiO3 continuously decreases to 0, suggesting that an oxidation reaction of H2 occurs, generating H2O.
【Innovation】 Achieved stable hydrogen production for 100 hours for the first time, and proposed the phenomenon of H2 being reoxidized to water by photocatalysis.
【Reflection】 It is recommended to read this alongside the 2020 Nature article on EQE=96% photocatalytic complete water splitting, which highlights Kazunari Domen’s coherent research approach, rigorous observations, and meticulous thinking, along with his 40 years of dedication to addressing fundamental scientific questions, which is truly admirable!