EGFR
In the Chinese patient population, EGFR is the most common driver gene mutation type in non-small cell lung cancer and is also one of the gene types with relatively good efficacy.
However, even for this type of non-small cell lung cancer, there are still some “blind spots” in targeted therapy, such as certain difficult-to-treat mutations (e.g., ex20ins) and resistance mutations (C797S). This year, multiple studies presented new data at the World Lung Cancer Conference targeting these difficult subtypes.
1
Fumetinib: Response Rate Approaching 80%
According to data shared at this year’s World Lung Cancer Conference from the phase Ib FAVOUR trial (NCT04858958), treatment with Fumetinib for patients with non-small cell lung cancer carrying EGFR exon 20 insertion (ex20ins) mutations achieved an overall response rate as high as 78.6% in some cohorts.
The report indicated that among treatment-naive patients, those receiving Fumetinib 240 mg QD had an overall response rate of 78.6%, with a preliminary median duration of response of 15.2 months; additionally, for previously treated patients who had undergone frontline therapy, the overall response rates in the 240 mg and 160 mg dose groups were 46.2% and 38.5%, respectively.
In previous studies, various strategies, including dose doubling, were attempted with Osimertinib to “overcome” the EGFR ex20ins difficult mutation, but the efficacy was only moderate (the overall response rate for high-dose Osimertinib in patients with EGFR ex20ins mutations was about 24%~28%), and the incidence of adverse events significantly increased (diarrhea was the most common, with an incidence of >70%), leading to poor patient tolerance. The data released this time also suggests that Fumetinib has the potential to surpass Osimertinib in this indication!
2
Rociletinib: Response Rate for Rare Mutations Exceeds 40%
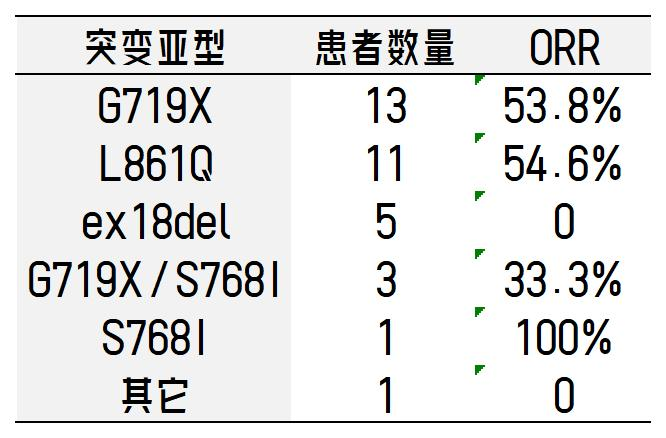
Rociletinib is a third-generation EGFR inhibitor, and at this year’s World Lung Cancer Conference, new clinical trial data for patients with rare mutations were presented. The trial included patients with rare EGFR mutations (G719X, S768I, L861Q, G719X+S768I, G719X+L861Q, L861Q+S768I, L747S, S720A, E709A, ex18del, etc.) and without common EGFR mutations (ex19del, L858R, T790M, ex20ins) in non-small cell lung cancer.
According to the report, the overall response rate was 44.1%, and the disease control rate was 85.3%; the median progression-free survival was 7.69 months. The response rates by mutation subtype are as follows:
3
HER3-DXd: Nearly 30% Response Rate in Osimertinib-Resistant Patients
At this year’s World Lung Cancer Conference, the antibody-drug conjugate (ADC) targeting the HER3 site, Patritumab Deruxtecan (HER3-DXd, U3-1402), updated its efficacy in patients with EGFR-mutated non-small cell lung cancer. Among the 209 patients who were resistant to third-generation EGFR inhibitors and platinum-based chemotherapy, the overall response rate was nearly 30%!
According to the report, in the phase II HERTHENA-Lung01 trial, a total of 225 patients were included, all of whom had previously received treatment with EGFR inhibitors and platinum-based chemotherapy. The overall response rate for those receiving HER3-DXd treatment was 29.8%, and the disease control rate reached 73.8%, with a median duration of response of 6.4 months and a median progression-free survival of 5.5 months.
Among these patients, 209 had already received treatment with third-generation EGFR inhibitors and platinum-based chemotherapy. In this subgroup, the overall response rate for HER3-DXd was still 29.2%, and the disease control rate reached 72.7%! The median progression-free survival for these patients was 5.5 months, and the median overall survival was 11.9 months.
MET
As an oncogene, MET abnormalities account for approximately 2%~4% of all non-small cell lung cancers, with common types including MET exon 14 skipping mutations and MET amplification.
1
Savolitinib: Nearly 60% Response Rate in Treatment-Naive Patients
This year’s World Lung Cancer Conference presented phase IIIb clinical trial data evaluating the efficacy of Savolitinib as first-line treatment for non-small cell lung cancer patients with MET exon 14 skipping mutations. Cohort 2 included 87 patients, of whom 84 completed at least one efficacy assessment.
According to the report, patients treated with Savolitinib had an overall response rate of 59.5%, and the disease control rate was as high as 95.2%; the median progression-free survival exceeded 1 year, reaching 12.6 months.
This trial shows that targeted therapy can achieve relatively ideal efficacy for treatment-naive MET mutation patients, and patients seeking better efficacy with fewer side effects can consider starting clinical trials or new drug treatments once MET abnormalities are confirmed.
2
JNJ-6372: Nearly 60% Response Rate in Treatment-Naive Patients
According to the results of the phase I CHRYSALIS trial presented at this year’s World Lung Cancer Conference, treatment with Evantuzumab (JNJ-6372) for primary MET exon 14 skipping mutation non-small cell lung cancer patients showed good efficacy.
According to the report, a total of 97 patients were enrolled, all of whom had primary MET mutations. The overall response rate for patients treated with Evantuzumab was 33%; among them, the overall response rate for treatment-naive patients was 56%; the overall response rate for patients who had not received MET inhibitor treatment (but may have received chemotherapy) was 46%, while the overall response rate for patients who had previously used MET inhibitors was 19%.
The median duration of response was 11.2 months; the overall clinical benefit rate was 69%, with a clinical benefit rate of 88% for treatment-naive patients.
ROS1
ROS1 is detected in approximately 2% of non-small cell lung cancers and is also known as the “diamond mutation.” This year’s World Lung Cancer Conference had relatively few studies targeting this site, but the results were very significant!
1
Repotrectinib: 59% Response Rate in Resistant Patients
According to updated data from the phase I/II TRIDENT-1 trial (NCT03093116) presented at this year’s World Lung Cancer Conference, Repotrectinib has durable efficacy for both treatment-naive and resistant ROS1-positive non-small cell lung cancer, and it is particularly effective for brain metastases!
According to the report, in treatment-naive ROS1-positive non-small cell lung cancer patients (71 cases), the overall response rate for Repotrectinib treatment reached 79%! Additionally, the median duration of response was 34.1 months, and the median progression-free survival was 35.7 months. The 12-month and 18-month progression-free survival rates were 77% and 70%, respectively, while the 12-month and 18-month survival rates were 91% and 88%.
For patients who had received one targeted drug treatment but had not used chemotherapy, the overall response rate for Repotrectinib treatment was 38%; the median duration of response was 14.8 months, the median progression-free survival was 9.0 months, and the median overall survival was 25.1 months. The 12-month progression-free survival and survival rates were 41% and 69%, respectively.
Moreover, for patients with the G2032R resistance mutation (one of the most common resistance mutation types after first-generation ROS1 inhibitor treatment), the overall response rate for Repotrectinib treatment was 59%; the median duration of response was 7.6 months, and the median progression-free survival was 9.2 months.
For patients with brain metastases, Repotrectinib also showed good efficacy. Among treatment-naive patients, the intracranial lesion response rate for Repotrectinib treatment was 89%; for previously treated patients, the intracranial lesion response rate was 38%. Researchers believe that besides treatment, the Repotrectinib regimen can also delay the occurrence of brain metastases in patients who have not yet developed them or prevent the occurrence of brain metastases.
2
Entrectinib vs. Crizotinib: Doubling Progression-Free Survival!
In recent years, Entrectinib, another first-generation ROS1 inhibitor, has been approved, significantly improving the efficacy for ROS1-positive non-small cell lung cancer! At this year’s World Lung Cancer Conference, a study targeting the Asian patient population quantified this efficacy improvement, allowing us to intuitively feel the durability of Entrectinib’s efficacy.
According to the report, patients treated with Entrectinib had a median progression-free survival of 39.4 months; this means that these patients’ lesions continued to shrink or did not increase for over 3 years! In contrast, the median progression-free survival for patients treated with Crizotinib was 15.9 months.
Additionally, the study also analyzed the overall survival of the two groups of patients receiving treatment, but over 50% of patients treated with Entrectinib remained alive, making it impossible to determine the median overall survival; while the median overall survival for patients treated with Crizotinib was 44.2 months.
KRAS
When discussing difficult mutations in non-small cell lung cancer, KRAS undoubtedly holds a significant position. The detection rate of this type of driver gene mutation in non-small cell lung cancer can reach 20%~40%, and carriers are resistant to multiple targeted therapies, resulting in poor survival rates.
However, with the increasing number of KRAS inhibitors entering clinical use, patients with this mutation, once considered “undruggable,” now have hope for long-term survival.
1
MRTX849: Long-Term Survival Despite Multi-Line Resistance
At this year’s World Lung Cancer Conference, Adagrasib (MRTX849) presented the latest long-term follow-up data, with a follow-up time exceeding 2 years.
According to the report, at a median follow-up of 26.9 months, patients treated with Adagrasib had an overall response rate of 43.0%, with a median duration of response of 12.4 months; the median progression-free survival was 6.9 months; the 1-year progression-free survival rate was 35.0%; and the median overall survival was 14.1 months, with 1-year and 2-year survival rates of 52.8% and 31.3%, respectively.
Moreover, patients with brain metastases also achieved good treatment outcomes: the median overall survival for patients with baseline brain metastases reached 18.7 months.
2
JDQ443: Response Rate Exceeds 30% After Targeted Resistance
At this year’s World Lung Cancer Conference, a new drug provided hope for patients regarding sequential treatment with KRAS inhibitors.
JDQ443 is a KRAS G12C inhibitor, and its partner TNO155 is an SHP2 inhibitor. According to the report, among 12 patients with non-small cell lung cancer who had previously received KRAS G12C inhibitor treatment, the combination of JDQ443+TNO155 achieved an overall response rate of 33.3%, and a disease control rate of 66.7%.
HER2
In the past decade, research on HER2-positive non-small cell lung cancer has progressed slowly. However, with the advent of several antibody-drug conjugates (ADCs), there has finally been a breakthrough in efficacy for these cancer patients. At this year’s World Lung Cancer Conference, multiple clinical trial data were released.
1
DS-8201: Response Rate Exceeds 50%
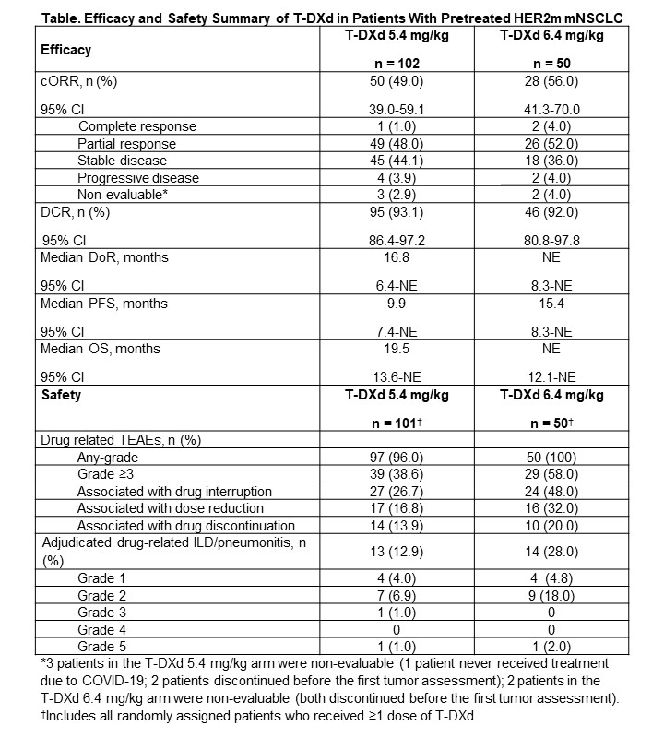
The DESTINY-Lung02 trial analyzed the efficacy of Trastuzumab Deruxtecan (DS-8201, Enhertu) in treating previously treated HER2-mutated metastatic non-small cell lung cancer with two different dosing regimens.
According to the report, patients treated with 5.4 mg/kg of Trastuzumab Deruxtecan had an overall response rate of 49.0%, with a median duration of response of 16.8 months, a median progression-free survival of 9.9 months, and a median treatment duration of 7.7 months;
Patients treated with 6.4 mg/kg of Trastuzumab Deruxtecan had an overall response rate of 56.0%, with the median duration of response being unassessable, a median progression-free survival of 15.4 months, and a median treatment duration of 8.3 months.
2
Poziotinib: Effective in Multi-Drug Resistant Patients
The phase II ZENITH20 trial analyzed the efficacy of Poziotinib in treating HER2 ex20ins mutation patients who had previously received at least two systemic treatments. All patients had undergone platinum-based chemotherapy, 78% had received immunotherapy, 36% had received HER2 antibodies or ADCs, and 17% had received TKIs.
According to the report, the overall response rate for treated patients was 30%, with a median duration of response of 5.5 months and a median progression-free survival of 5.6 months.
Among them, patients who had previously received platinum + any systemic treatment had an overall response rate of 31.3%; patients who had previously received platinum + docetaxel treatment had an overall response rate of 30.8%; patients who had previously received platinum + HER2 antibody treatment had an overall response rate of 24.0%; and patients who had previously received platinum + TKI treatment had an overall response rate of 54.5%.
New Drugs and Clinical Trials Bring New Hope for Long-Term Survival!
In recent years, the number of new lung cancer patients in China has been rising sharply, reaching over 815,000 in 2020, with nearly 70% of patients diagnosed at an advanced stage.
With the improvement of medical standards, the 5-year survival rate for cancer patients in China has increased by about 10% over the past decade, and the survival period for lung cancer patients has also improved to some extent. The widespread use of new targeted therapies and clinical trials has undoubtedly made a significant contribution, allowing many late-stage patients to achieve “long-term survival with tumors.”
Source: Global Oncology Medical News
Disclaimer: This article is reproduced for learning and communication purposes only, not for profit. If you like this article, you can follow the original author. Copyright belongs to the original author. If there are any copyright issues with the content or images, please contact us, and we will delete them immediately.