On April 11, 2025, the Zhu Chaozhe team from the National Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University published a study titled “Evaluation of scalp-based targeting methods of DLPFC for TMS therapy” in the journal Transcranial Magnetic Stimulation. This study systematically compared four scalp targeting methods (EEG Cap, Beam F3, CPC F3, and adjusted Beam F3) for Transcranial Magnetic Stimulation (TMS) treatment of depression. The results indicated that the CPC F3 method demonstrated superior overall performance, providing important scientific evidence for the selection of clinical treatment targeting schemes.



There are over 300 million patients with depression worldwide who urgently need effective treatment methods. TMS, as a non-invasive therapy, has been widely applied in clinical settings. The key issue affecting efficacy is how to position the coil to accurately target the left dorsolateral prefrontal cortex (DLPFC). Existing magnetic resonance neuro-navigation technologies face challenges such as high costs and complex operations. In contrast, scalp-based targeting methods offer economic and operational advantages and have been recommended as essential skills for TMS practitioners in clinical practice guidelines. Currently, there are various clinical scalp targeting methods for DLPFC, including EEG Cap, Beam F3, CPC F3, and adjusted Beam F3, but it remains unclear which method performs best.
To address this issue, the research team established a comprehensive evaluation system that includes targeting accuracy, reliability, and speed, using the peak location of the functionally negative correlation with the anterior cingulate cortex (MNI coordinates [X-42 Y+44 Z+30]) as the target brain area, and conducted experimental research. The experimental results showed that the CPC F3 method performed best in terms of targeting accuracy (see Figure 2). Its average targeting error was 4.16 mm (notably, the reported magnetic resonance neuro-navigation targeting error in literature 8 is approximately 3.6 mm), significantly lower than the EEG Cap method (7.67 mm), Beam F3 (6.08 mm), and adjusted Beam F3 method (6.93 mm). The electric field simulation results indicated that the average electric field strength induced in the target area by CPC F3 was also slightly higher than the other three methods. Additionally, cross-ethnic validation showed that the accuracy advantage of CPC F3 was unaffected. In terms of targeting reliability, the CPC F3 method also performed outstandingly, with inter-technician and intra-technician targeting deviations of 3.30 mm and 2.32 mm, respectively, significantly lower than other methods. This characteristic is particularly important for TMS treatments that require multiple sessions of stimulation. In terms of targeting speed, the EEG Cap method was the fastest among the four methods, averaging 80 seconds, but it requires purchasing a suitable EEG cap based on the patient’s head circumference, which imposes certain requirements on clinical equipment. CPC F3, with an average measurement time of 105 seconds, ranked second and can be completed with just a regular measuring tape, offering good operational flexibility, making it more applicable in high patient volume and limited equipment medical settings. Beam F3 and adjusted Beam F3 methods require software or website assistance, resulting in significantly longer measurement times (averaging 140 seconds).
In addition to examining the targeting performance for the target brain area, this study also directly compared the targeting performance of several methods for the scalp F3 position. The experimental results similarly indicated that CPC F3 performed best (see supplementary materials of the article). Recently, other studies have also focused on this clinical targeting issue (see literature 3). This study also found that the targeting accuracy of CPC F3 was significantly superior to that of Beam F3, and its targeting accuracy was not affected by head geometry, subject age, gender, or clinical phenotype, and the measurement results were unbiased in spatial distribution. The authors of this study evaluated the CPC F3 method: “This not only demonstrates the higher validity and reliability of the SGP method, but also shows that SGP is a very promising alternative TMS coil positioning approach in a general sense. It may finally allow breaking out of the coarse 10/10 electrode grid that has been used in TMS research for decades.” (Note: The SGP system includes two subsystems: scalp positioning and scalp orientation, where scalp positioning uses the CPC system, and coil orientation is represented by angles within the scalp plane).



This research was led by the National Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University, in collaboration with Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine and Shanghai Mental Health Center. The co-first authors of the paper are the graduated PhD Jiang Yihan (currently an assistant researcher at the School of Psychology and Cognitive Science at Beijing Language and Culture University) and the current PhD student Chen Yuanyuan, with significant contributions from PhD students Wei Lijiang, Liu Farui, Zheng Zeqing, and others. The corresponding authors are Professor Zhu Chaozhe and Professor Niu Chuanxin (Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine). This research was jointly funded by the National Natural Science Foundation of China and the doctoral research startup fund of Beijing Language and Culture University.
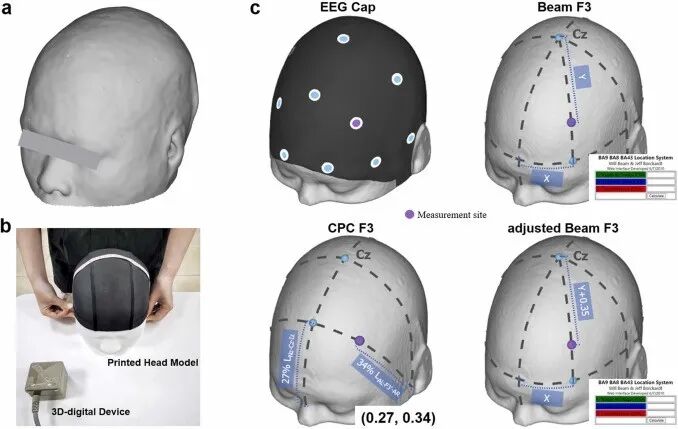
Figure 1. Evaluation experiment. (a) Example of a 3D printed head model; (b) Example of the experimental scene; (c) Schematic diagram of the measurement steps for the four scalp positioning methods.
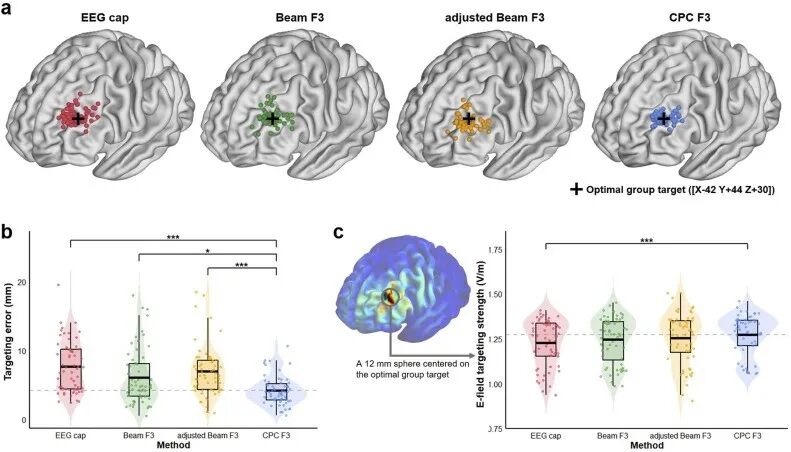
Figure 2. Results of positioning location records and comparison of positioning accuracy.
Other Related Studies


1. Jiang, Y., Du, B., Chen, Y., Wei, L., Zhang, Z., Cao, Z., Xie, C., Li, Q., Cai, Z., Li, Z., & Zhu, C. (2022). A scalp-measurement based parameter space: Towards locating TMS coils in a clinically-friendly way. Brain Stimulation, 15(4), 924–926. https://doi.org/10.1016/j.brs.2022.06.001
2. Liu, F., Zhang, Z., Chen, Y., Wei, L., Xu, Y., Li, Z., & Zhu, C. (2023). MNI2CPC: A probabilistic cortex-to-scalp mapping for non-invasive brain stimulation targeting. Brain Stimulation, 16(6), 1733–1742. https://doi.org/10.1016/j.brs.2023.11.011
3. Zhen Li, Alexander T. Sack, Felix Duecker, Finding F3: A comparative analysis of scalp-based methods for TMS coil positioning, Transcranial Magnetic Stimulation, Volume 2, 2025, 100083, ISSN 3050-5291, https://doi.org/10.1016/j.transm.2024.100083.
4. Xiao, X., Yu, X., Zhang, Z., Zhao, Y., Jiang, Y., Li, Z., Yang, Y., & Zhu, C. (2018). Transcranial brain atlas. Science Advances, 4(9). https://doi.org/10.1126/sciadv.aar6904
5. Zhang, Z., Li, Z., Xiao, X., Zhao, Y., Zuo, X.-N., & Zhu, C. (2021). Transcranial brain atlas for school-aged children and adolescents. Brain Stimulation, 14(4), 895–905. https://doi.org/10.1016/j.brs.2021.05.004
6. Jiang, Y., Li, Z., Zhao, Y., Xiao, X., Zhang, W., Sun, P., Yang, Y., & Zhu, C. (2020). Targeting brain functions from the scalp: Transcranial brain atlas based on large-scale fMRI data synthesis. NeuroImage, 210, 116550. https://doi.org/10.1016/j.neuroimage.2020.116550
7. Wei, L., Zhao, Y., Liu, F., Chen, Y., Xu, Y., Li, Z., & Zhu, C. (2025). Transcranial brain atlas based on photon measurement density function in a triple-parameter standard channel space. NeuroImage, 307, 121026. https://doi.org/10.1016/j.neuroimage.2025.121026
8. Nieminen, A. E., Nieminen, J. O., Stenroos, M., Novikov, P., Nazarova, M., Vaalto, S., Nikulin, V., & Ilmoniemi, R. J. (2022). Accuracy and precision of navigated transcranial magnetic stimulation. Journal of neural engineering, 19(6), 10.1088/1741-2552/aca71a. https://doi.org/10.1088/1741-2552/aca71a