Introduction: Sexual desire, arousal, and climax are mediated by complex, yet not fully understood, interactions of the somatic and autonomic nervous systems operating at both central and peripheral levels. Disruption of endocrine, neural, or vascular responses caused by aging, medical illnesses, neurological diseases, surgery, or medications can lead to sexual dysfunction, significantly affecting the quality of life of patients.
Objective: This narrative review aims to describe the role of the central nervous system in human sexual behavior.
Methods: A literature search was conducted using PubMed for all literature up to June 2018, analyzing studies related to the neurobiological and neurophysiological basis of human sexual behavior.
Results: Sexual behavior is regulated by subcortical structures (such as the hypothalamus, brainstem, and spinal cord) and several cortical areas that act as orchestrators finely tuning this primal, complex, and variable behavior. At the central level, dopaminergic and serotonergic systems appear to play important roles in various factors of sexual response, although adrenergic, cholinergic, and other neuropeptide systems may also be involved.
Conclusion: Providing healthcare professionals with information about sexual behavior may overcome useless and sometimes dangerous barriers and improve patient management, as sexual health is considered one of the most important aspects of an individual’s quality of life.
Sexual response is a key physiological condition deeply rooted in the entire species. The study of animal sexual behavior is a complex topic that can be approached from various angles, as it relies on the interactions between neural, endocrine, and genetic factors. In humans, sexual behavior is influenced by cultural environments that require dynamic behavioral adaptations. Thus, multi-system interactions are needed to ensure human characteristics and more complex typical functions. The neural structures involved in sexual behavior are located throughout the nervous system, including both central and peripheral parts. The detection of multimodal sexual stimuli involves sensory processing combined with experiences to trigger autonomic nervous and appropriate motor responses under overwhelming cognitive control. To date, 2016; Clark and Hatfield, 1989; Peterson and Hyde, 2010).
This difference in sexual attitudes between males and females can be partially attributed to the dimorphic anatomical substrate located in the genitalia and the nervous system, as well as the different hormonal distributions (Hausmann, 2017; MacLusky and Naftolin, 1981; McEwen and Milner, 2017).
However, despite several neuroimaging studies providing new insights into the potential mechanisms of human sexual behavior, some puzzles remain unsolved.
According to Masters and Johnson, the physiological responses during sexual stimulation include sequential phases of excitement, plateau, orgasm, and resolution (Masters & Johnson, 1966).
Later, Kaplan’s triphasic model (consisting of three phases, which are considered inevitable and interconnected: desire, excitement, and orgasm) gained stronger appeal due to its clinical relevance (Kaplan, 1974).
Sexual desire is typically defined as the motivation to respond sexually to relevant internal and external cues, including sexual thoughts, fantasies, and engagement in sexual behavior (Buss & Schmitt, 1993).
Additionally, it is influenced by many factors such as attitudes, opportunities and/or partner availability, emotions, and health status. During the excitement phase, the body prepares for intercourse, which results from any erotic physical or mental stimulation leading to sexual arousal. Sexual arousal is closely related to sexual desire and can be defined in subjective terms (i.e., feeling sexually excited) and physiological terms (i.e., genital vascular engorgement and swelling).
In males, physiological arousal begins with erection, a reflex event driven by sensory signals transmitted by the dorsal nerve of the penis after stimulation of free nerve endings located in the penis and glans. The hemodynamic characteristics of penile blood flow during erection are caused by engorgement of the corpora cavernosa due to vasodilation induced by the release of nitric oxide from endothelial cells stimulated by parasympathetic nerves projecting from the pelvic nerve. On the other hand, penile detumescence is mediated by the pelvic nerves, cavernous nerves, and pudendal nerves of the sympathetic nervous system, along with several vasoconstrictive factors. Erection depends on responses to tactile, visual, imaginative, and olfactory inputs, controlled by spinal and supraspinal mechanisms. Reflexogenic and psychogenic stimuli are likely to interact synergistically via sacral parasympathetic pathways. Although little is known about the supraspinal events involved in erectile function and they are primarily based on animal models, the hypothalamus and limbic pathways appear to play key roles in erection.
Female arousal relies on similar mechanisms; however, sexual excitement is cyclical with the menstrual cycle. The hemodynamic response of the clitoris after sexual stimulation is controlled by the autonomic nervous system. During sexual arousal, the vestibular glands located on either side of the vaginal opening produce mucus, which lubricates the area along with vaginal secretions, making intercourse more comfortable (Yucel et al., 2004).
Moreover, the excitement phase leads to increases in heart rate, respiratory rate, and blood pressure, triggered by several nuclei, the brainstem, and the medial preoptic area of the hypothalamus. Cutaneous vasodilation leads to sexual flushing (mainly in the chest and neck), which usually disappears shortly after orgasm. Orgasm is characterized by the rapid contraction of the muscles surrounding the anus and primary sexual organs, accompanied by euphoria and further increases in heart rate (Masters & Johnson, 1966).
Nevertheless, the peak of the arousal phase cannot be considered a simple sequence of physical events. In fact, it is well known that sexual dysfunction (i.e., anorgasmia) severely affects an individual’s quality of life and psychology.
The aim of this review is to provide useful information to healthcare professionals about human sexual behavior and its neural correlates for better patient management. Our main argument is that a better understanding of human sexual behavior may provide a universal perspective to understand (a) how the brain generates it for the fortunate in daily life, (b) how the brain fails in the unfortunate, and hopefully (c) good habits to improve patients’ quality of life.
1. Neuroanatomy of Human Sexual Behavior
Sexual behavior relies on the processing of sexual stimuli that allow individuals to enter the human sexual cycle. From an evolutionary perspective, this is a fundamental behavior as it supports reproductive interactions that are crucial for biological adaptation and species self-preservation. However, the impact of sex on human daily life extends far beyond its prototypical purpose. Over the years, multiple pieces of evidence have confirmed that regular sexual activity has positive effects on both physical and mental health. Therefore, it is important for physicians and other healthcare professionals to understand the neural mechanisms behind sexual behavior. Each stage of the human sexual cycle involves neural structures from the cerebral cortex to the peripheral nervous system.
Different unimodal specific sexual cues are processed in the central nervous system, where complex integrative activities lead to autonomic and voluntary responses. The way sexual information flows through the brain reflects the program of goal-directed behavior, known as the “sexual pleasure cycle” (Georgiadis & Kringelbach, 2012; Georgiadis, Kringelbach, & Pfaus, 2012).
Sexual desire and pleasure experience are key components of the sexual pleasure cycle, and their experience depends on the dopaminergic neurons of the reward system primarily located in the midbrain (substantia nigra – SNc, ventral tegmental area – VTA) and the interacting opioid – endocannabinoid system.
Moreover, sexual behavior requires implicit sensory stimuli that are evaluated as sexually salient and compared with past experiences, thereby triggering the induced motivational state. The respective anatomical substrates are the limbic forebrain structures, such as the hypothalamus, amygdala, hippocampus, and bed nucleus of the stria terminalis, involved in motivational states and emotional processing. Different sensory cues are integrated unconsciously by limbic structures, thereby triggering typical autonomic responses (heart rate, blood pressure, and respiratory rate increase with brainstem structures). Nevertheless, all stages of the human sexual cycle involve complex conscious awareness, primarily characterized by the cerebral cortex (see Table 1 for a summary of brain structures regulating human sexual behavior). Georgiadis (2015) provides a comprehensive review of the role of the cerebral cortex in sexual behavior.
2. Neuroanatomy of Human Sexual Behavior
Sexual behavior relies on the processing of sexual stimuli that allow individuals to enter the human sexual cycle. From an evolutionary perspective, this is a fundamental behavior as it supports reproductive interactions that are crucial for biological adaptation and species self-preservation. However, the impact of sex on human daily life extends far beyond its prototypical purpose. Over the years, multiple pieces of evidence have confirmed that regular sexual activity has positive effects on both physical and mental health. Therefore, it is important for physicians and other healthcare professionals to understand the neural mechanisms behind sexual behavior. Each stage of the human sexual cycle involves neural structures from the cerebral cortex to the peripheral nervous system.
Different unimodal specific sexual cues are processed in the central nervous system, where complex integrative activities lead to autonomic and voluntary responses. The way sexual information flows through the brain reflects the program of goal-directed behavior, known as the “sexual pleasure cycle” (Georgiadis & Kringelbach, 2012; Georgiadis, Kringelbach, & Pfaus, 2012).
Sexual desire and pleasure experience are key components of the sexual pleasure cycle, and their experience depends on the dopaminergic neurons of the reward system primarily located in the midbrain (substantia nigra – SNc, ventral tegmental area – VTA) and the interacting opioid – endocannabinoid system.
Moreover, sexual behavior requires implicit sensory stimuli that are evaluated as sexually salient and compared with past experiences, thereby triggering the induced motivational state. The respective anatomical substrates are the limbic forebrain structures, such as the hypothalamus, amygdala, hippocampus, and bed nucleus of the stria terminalis, involved in motivational states and emotional processing. Different sensory cues are integrated unconsciously by limbic structures, thereby triggering typical autonomic responses (heart rate, blood pressure, and respiratory rate increase with brainstem structures). Nevertheless, all stages of the human sexual cycle involve complex conscious awareness, primarily characterized by the cerebral cortex (see Table 1 for a summary of brain structures regulating human sexual behavior). Georgiadis (2015) provides a comprehensive review of the role of the cerebral cortex in sexual behavior.
Table 1 Summary of Brain Regions Involved in Human Sexual Behavior
| Brain Region | Sexual Function |
|---|---|
| Reward System |
Triggering Sexual Motivation Mate Selection |
| Thalamus | Transmitting Erotic Stimuli from the Spinal Cord |
| Hypothalamus |
Coordinating Autonomic Events in Sexual Behavior Mate Selection |
| Amygdala |
Imbuing Incoming Erotic Stimuli with Emotional Significance Mate Selection Regulating Sexual Desire |
| Bed Nucleus | Regulating Sexual Desire |
| Prefrontal Cortex |
Inhibiting the Initiation of Sexual Behavior Regulating Sexual Desire |
| Cingulate Cortex |
Processing Sexual Stimuli in Conflict Situations Sexual Desire Module |
| Insula |
Awareness of Engorgement of Erect Organs Regulating Sexual Desire |
Thus, the sensory-motor cortex participates in triggering autonomic movements and genital sensations during the intercourse process, while higher associative areas play critical roles in erotic mental imagery and inhibition of sexual impulses. On the other hand, the spinal cord is mainly involved in erection of the penis and clitoris, lubrication of the vagina and penis glands, and rhythmic contractions of perineal muscles. Other important areas, such as the nucleus paragigantocellularis (nPG1), locus coeruleus (LC), raphe nuclei, and periaqueductal gray area located in the brainstem, are closely related to the spinal cord and primarily involved in erection and ejaculation. Finally, regarding the autonomic system, it should be noted that the parasympathetic nervous system is related to erection and female lubrication, while the sympathetic one is involved in ejaculation and orgasm. The interaction between neuropeptides involved in vascular tone, such as vasoactive intestinal peptide (VIP), which acts as a vasodilator, and neuropeptide Y, which induces venous vasoconstriction, leads to female lubrication. This process results in an increase in interstitial fluid, leading to vaginal lubrication (Levin, 2003).
3.1. Reward System
Similar to other behaviors, sexual behavior also has a beginning, process, and end. All organisms engaging in sexual behavior share a set of common principles and endpoints defining the behavior itself and specific neural mechanisms that make it successful. So far, a behavior is defined as successful when it is flexible enough to maximize rewards and minimize adverse outcomes.
Allowing sexual responses to become routine or automated neural pathways that induce plasticity and reshape behavior is related to positive sexual reinforcement, which includes dopaminergic release structures of the reward system. The aforementioned pathways have been confirmed in animals and may also exist in humans, playing a decisive role in shaping individual behavior adaptively in response to environmental changes (Pfaus, Kippin, & Coria-Avila, 2003).
In neuroscience, the reward system consists of the VTA, located anterior to the dorsal raphe nucleus and periaqueductal gray; moreover, it projects widely to the nucleus accumbens (NAc, ventral striatum) and prefrontal cortex. The first pathway constitutes the dopaminergic mesolimbic pathway, while the latter constitutes the dopaminergic cortical-limbic pathway. Additionally, at the midbrain level, dopaminergic neurons from the SNC primarily project to the dorsal striatum. Neurons belonging to the reward system exhibit spontaneous tonic and phasic activity. Spontaneous tonic peaks are thought to maintain the basal level of extracellular dopamine, constituting “background activity,” while phasic activity leads to sudden increases in dopamine levels, which are related to reward prediction errors (Schultz, 2010).
Notably, several structures projecting to the VTA regulate its firing pattern through different neurotransmitters, as phasic burst firing depends on presynaptic activity (Floresco, West, Ash, Moore, and Grace, 2003; Lecca, Melis, Luchicchi, Muntoni, and Pistis, 2012; Pignatelli and Bonci, 2015).
In particular, GABAergic neurons from the NAc, ventral pallidum, and interstitial nucleus of the anterior hypothalamus regulate the firing pattern of VTA neurons, exhibiting modulatory effects that can shift tonic activity to bursting patterns. Conversely, glutamatergic inputs from the prefrontal cortex, bed nucleus of the stria terminalis, and pedunculopontine nucleus (exerting excitatory effects) indeed lead to burst firing. In fact, a recent fMRI study indicated that the OFC encodes the value and identity of rewards, while the activity of the vmPFC seems to be more involved in the categorization of stimuli across reward categories (Howard, Gottfried, Tobler, and Kahnt, 2015).
Therefore, reward shapes behavior, as several cortical regions attribute value to incoming predictive stimuli leading to adaptive choices.
Reward processing is further modulated by the endocannabinoid system, which produces lipid neuromodulators whose specific receptors (CB1) have been found in the VTA, hippocampus, amygdala, and hypothalamus (for a broad review of the effects of the endocannabinoid system on behavior, see Sagheddu, Muntoni, Pistis, and Melis, 2015).
Several studies have pointed out that dopamine (DA) is a major player in triggering sexual motivation, suggesting that increased DA levels in structures belonging to the reward system would lead to a behavioral shift towards hypersexuality. Notably, sexual stimuli are associated with many neural substrates involved in reward processing and complex cognitive functions, such as the NAc, caudate nucleus, insula, thalamus, orbitofrontal cortex – OFC, and dorsal anterior cingulate cortex – dACC, including decision-making and salience (Haber, 2011; Haber & Knutson, 2009).
In addition to the well-known role of the NAc (i.e., a structure in the ventral striatum) in reward processing, various neuroimaging studies have shown increased activity in the ventral striatum in response to erotic stimuli (Brand, Snagowski, Laier, and Maderwald, 2016; Childress et al., 2008).
Notably, the NAc is part of a broad network of neural correlates of sexual arousal, and it has been reported to track sexual preferences of heterosexual, homosexual, and bisexual individuals (Safron et al., 2007, 2017, 2018).
Furthermore, Oei, Rombouts, Soeter, Gerven, and Both (2012) showed that DA stimulates activity in the NAc and dACC in response to subliminally perceived sexual stimuli, indicating that DA may influence sexual motivation at its earliest occurrence, which seems to occur outside of conscious awareness (Oei et al., 2012).
As a major structure of the reward system, the thalamus has traditionally been considered a relay center for sexual stimuli from the spinal cord to the cortex and subcortical structures. Thalamic electrical stimulation and deep brain stimulation have been shown to affect penile erection in primates (Robinson & Mishkin, 1968) and humans (Temel et al., 2004).
Moreover, several fMRI studies have associated increased thalamic activity with erotic visual stimuli (Park, Kang et al., 2001; Park, Seo et al., 2001; Redouté et al., 2000).
It has been reported that the thalamus facilitates the processing of sexual preferences and relays them to the temporal lobe, where they undergo complex behavioral changes. Thus, the thalamus is likely to play a key role in mate selection (Mutarelli, Omuro, and Adoni, 2006; Spinella, 2004), reinforcing the notion that the thalamus is not only a relay center from the spinal cord to the cortex but also an integrative center that is active throughout the phases of desire, arousal, and orgasm.
3.2. Subcortical Limbic Structures
3.2.1. Hypothalamus
The hypothalamus is the ventral part of the diencephalon located below the thalamus, composed of several nuclei with various functions. The hypothalamus occupies only 2% of brain volume but plays a crucial role in integrating endocrine, autonomic, and behavioral responses (Braak & Braak, 1992).
Hypothalamic releasing factors stimulate or inhibit the secretion of pituitary hormones, affecting body temperature, hunger, thirst, circadian rhythms, and sexual desire (Sam & Frohman, 2008).
Small lesions in the medial preoptic area/anterior hypothalamus (MPOA/AH) temporarily affect sexual desire in rats, while larger lesions have permanent effects on sexual behavior. Although MPOA stimulation regulates erection and coordinates autonomic events related to sexual response, the exact role of MPOA/AH in sexual behavior has long been controversial. Animal studies often report conflicting results regarding the dominant role of MPOA in sexual motivation and/or sexual completion (Davidson, 1966; Hughes, Everitt, & Herbert, 1990; Paredes, Tzschentke, and Nakach, 1998).
Recently, it has been proposed that MPOA likely receives information transmitted from the hippocampus via the lateral septum and from the amygdala via the bed nucleus of the stria terminalis (BNST; Pfaus, 2009).
Signals are further processed by projections to brainstem nuclei. Once sexual stimuli in the MPOA are integrated with information from the ventromedial nucleus, suprachiasmatic nucleus, arcuate nucleus, and ventral anterior nucleus, sexual motivation is triggered.
In a recent fMRI study, Brunetti et al. (2008) found a significant correlation between activation of the bilateral hypothalamus and deep sexual identity (DSI), defined as a multi-faceted, constant system combining biological, psychological, and cultural aspects of sexual identity (Brunetti et al., 2008).
More specifically, after revalidation of the Franck drawing completion test introduced by Olivetti Belardinelli (Olivetti Belardinelli, 1994), DSI was assessed, leading to a certain degree of consistency between self-reports and psychological identity when subjects were exposed to erotic stimuli (Brunetti et al., 2008).
Considering that DSI is interpreted as individual differences in sexual psychological attitudes, the significant positive correlation between BOLD activity in the bilateral hypothalamus and DSI (the higher the hypothalamic activation, the greater the consistency between self-reports and male psychological identity – the personal self-presentation as male) suggests that the hypothalamus may be the anatomical basis for individual differences in certain characteristics of sexual behavior. However, we consider this hypothesis to be merely speculative, as the current literature lacks stronger evidence.
Moreover, the hypothalamus participates in penile erection through two of its nuclei, the dorsal medial hypothalamus (DMHN) and the ventromedial nucleus (VMHN). DMN projects to the midbrain reticular formation through the central and dorsal gray matter. Both DMN and VMN reach the lumbar-sacral autonomic centers involved in penile erection via the dorsolateral funiculus of the spinal cord. Interestingly, the hypothalamic nuclei receive information directly from the genital regions (Marson & McKenna, 1994; Rajaofetra et al., 1992; Sachs, 1995; Steers, 2000).
Additionally, it has been shown that the paraventricular nucleus (PVN) is activated during mating and orgasm (Komisaruk & Whipple, 2005); this may be related to its specific endocrine functions as it produces oxytocin, vasopressin, enkephalins, and dopamine (Argiolas & Melis, 1995).
In particular, oxytocin has been shown to trigger activity in structures of the reward system following sexual or socially relevant stimuli (Gregory, Cheng, Rupp, Sengelaub, and Heiman, 2015; Groppe et al., 2013; Scheele, Plota, Stoffel-Wagner, Maier, and Hurlemann, 2016; Scheele et al., 2013).
Moreover, it has been demonstrated in rats that sensory stimulation induces activity, especially in the neurons secreting oxytocin (Yanagimoto, Honda, Goto, and Negoro, 1996).
However, activation of the PVN during fMRI does not imply that oxytocin secretion occurs simultaneously, as fMRI cannot identify the neurons secreting oxytocin; therefore, this hypothesis remains speculative. Finally, the anterior hypothalamus plays a role in the regulation of typical male sexual behavior. In fact, the interstitial nucleus of the anterior hypothalamus 3 (INAH3) has been shown to be dimorphic in males and females, which indicates that sexual orientation has a biological basis, as it has been reported to be smaller in females and homosexual males.
3.2.2. Amygdala
The amygdala is a group of almond-shaped nuclei located deep within the medial temporal lobe of the brain of complex vertebrates, including humans. The extensive connections of the amygdala complex with cortical and subcortical structures indicate its important role in human sexual impulses (Baird, Wilson, Bladin, Saling, and Reutens, 2004; McKenna, 1999; Swanson and Petrovich, 1998).
In fact, bilateral lesions of the amygdala are responsible for abnormal sexual behavior (“hypersexual state”), as observed in Kluver-Bucy syndrome (Lanska, 2018).
For example, the oldest region of the amygdala, the cortical nucleus, participates in olfactory and pheromone processing as it receives inputs from the olfactory bulb and olfactory cortex, thereby playing an important role in the sexual behavior of larger animals. The amygdala is part of a large network of brain structures involved in emotional processing, a complex phenomenon that relies on decoding and integrating sensory stimuli and comparing incoming information with past experiences.
The amygdala is one of the main players in the social brain (Adolphs, Tranel, and Damasio, 1998; Adolphs, Tranel, Damasio, and Damasio, 1994), a system that shapes behavior in the context of contextual adaptation trends. Once stimuli gain emotional relevance, they are relayed from the amygdala to the prefrontal cortex and OFC. Additionally, the amygdala projects to the foundational structures of sexual behavior mentioned earlier, such as the hypothalamus and NAc. Therefore, the amygdala complex provides modulation of autonomic responses and complex cognitive functions.
Results from animal studies clearly indicate that the amygdala is a key structure mediating sexual behavior. In humans, evidence of the role of the amygdala in sexual function comes from lesion studies showing that stimulation of the amygdala can elicit orgasm-like pleasure (Baird, Wilson, Bladin, Saling, and Reutens, 2007).
Moreover, Baird and colleagues demonstrated that a significantly larger volume of the amygdala contralateral to the resection site was associated with improved sexual function postoperatively (Baird et al., 2004).
One hypothesis that could partially explain this relationship emphasizes the involvement of the amygdala in processing emotionally charged stimuli. In fact, the amygdala receives projections from unimodal sensory areas and performs complex integration related to the assessment of emotional aspects.
Finally, many fMRI studies have shown significant sex-related differences in amygdala function. In fact, increased amygdala activation has been found in males compared to females, even though the latter exhibit greater sexual arousal (Hamann, Herman, Nolan, and Wallen, 2004; Seok, Sohn, and Cheong, 2016).
Furthermore, morphological and functional differences in the amygdala have been confirmed in homosexual individuals, supporting the hypothesis that human sexual preferences are partially attributable to dimorphic structures (Poeppl, Langguth, Rupprecht, Laird, and Eickhoff, 2016).
In homosexuals, more amygdala activity related to preferences has been observed compared to heterosexual males, but it remains unclear whether this is a cause or consequence of their sexual behavior (Safron et al., 2007).
However, the same findings were not subsequently replicated (Safron et al., 2017).
Moreover, Wehrum et al. (2013) identified a common neural network that plays an important role in processing sexual stimuli involving the amygdala, insula, and thalamus, regardless of sex. On the other hand, in addition to these similarities, males exhibit overall stronger responses, which may reflect a more intense sexual response in males than in females (Wehrum et al., 2013).
3.2.3. Cortical Regions
Throughout evolution, sexual behavior has become increasingly complex, and while significant roles can certainly be attributed to the subcortical structures of the limbic system and several nuclei in the brainstem, the cerebral cortex plays a major role in adapting and shaping sexual behavior under social and cultural influences. In fact, several cortical regions (see Figure 1) are used to consciously process sexual stimuli and calculate the appropriate response to sexual desire. Here, we consider the roles of the prefrontal cortex, OFC, cingulate cortex, and insula in human sexual behavior.
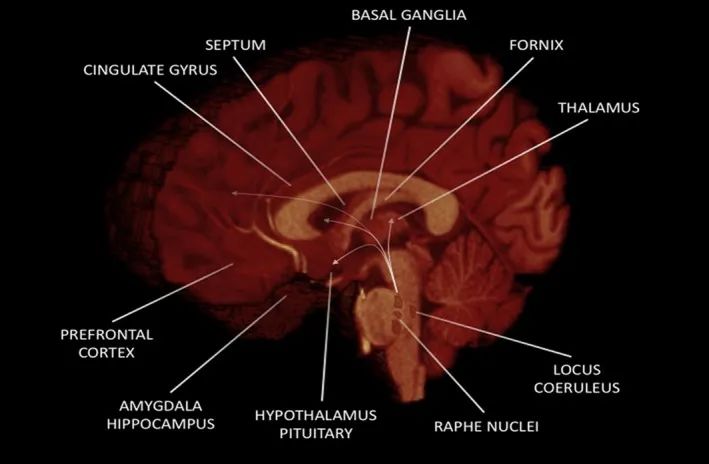
Figure 1 Major Brain Regions Involved in Human Sexual Behavior
3.2.4. Prefrontal and Orbitofrontal Cortex
The prefrontal cortex (PFC) is involved in planning complex cognitive behaviors, expressing personality, making decisions, and regulating appropriate social behavior (Amodio & Frith, 2006; Domenech & Koechlin, 2015; Frith & Dolan, 1996; Miller & Cohen, 2001).
This brain region plays a key role in shaping thoughts and actions according to internal goals to fully integrate and articulate stimuli in executive function outputs. Executive function involves the ability to distinguish conflicting thoughts, determine the good and bad, better and best, same and different future outcomes of current activities, strive towards clear goals, predict outcomes, base expectations on actions, and social “control” (the ability to inhibit impulses that, if not inhibited, may lead to socially unacceptable outcomes) (Kelly et al., 2004; Ridderinkhof, Wildenberg, Segalowitz, and Carter, 2004; Siddiqui, Chatterjee, Kumar, Siddiqui, and Goyal, 2008).
Sexual inhibition is believed to be an adaptive response serving both reproductive endpoints and social endpoints (i.e., keeping individuals out of trouble or allowing sufficient sexual satiety to occur during the “refractory period”). This should inhibit the complex and ongoing interaction between movement tendencies and planned and sustained actions. Regarding sexual behavior, it is believed that culture overlays moral values of “right” and “wrong” hierarchically, so that some “feel-good” behaviors are right and can be experienced without guilt, while others are wrong and carry the influence of guilt and/or legal rules against them. Thus, this type of inhibition represents an approach-avoidance conflict where the expectation of reward drives desire (2010; McNaughton, 2010).
Additionally, sexual inhibition can also be triggered by non-rewarding sexual components suppressing desire. Therefore, the “pro-sexual” nature of drugs such as alcohol or cocaine may exert their effects by inhibiting the ability of this suppressed sexual response. These inhibitory systems are located in the PFC and may inhibit the activation of mechanisms that shift attention and behavior to non-sexual stimuli or contexts. The opioid, endocannabinoid, and serotonin systems that mediate sexual reward states seem to be at least three neurochemical systems involved in sexual inhibition (Pfaus, 2009).
In patients with OFC lesions, hypersexuality is often observed, typically considered to be uninhibited sexual behavior. However, data from clinical studies and case reports have not been entirely consistent. Furthermore, lesions strictly occurring within the OFC do not recur as such. Therefore, disinhibited sexual behavior refers to frontal lobe lesions that impair the inhibitory processes generated by these regions (Baird et al., 2007; Béreau, 2018).
Moreover, the OFC region has been targeted for the pleasant bodily expressions and euphoria achieved at the peak of the sexual cycle. Finally, both the PFC and OFC are closely connected to the subcortical structures belonging to the reward system and are responsible for the cognitive filtering of sexual behavior (Schmidt et al., 2017).
3.3. Cingulate Cortex
The cingulate cortex is the most prominent part of the limbic system, encircling the corpus callosum from the rostral to the caudal part. It is divided into anterior, middle, and posterior regions. Many studies have pointed out the role of the ACC in sexual behavior. fMRI studies have revealed ACC activation in response to erotic stimuli, indicating that the ACC is involved in processing sexual stimuli in different contexts, enhancing decision-making through its outputs to motor-related areas and the periaqueductal gray (Arnow et al., 2002; Rauch et al., 1999; Redouté et al., 2000; Seok et al., 2016).
Additionally, Oei et al. (2012) showed that when sexual stimuli appear subliminally, dACC activation increases due to DA release, indicating its involvement in processing subliminal stimuli (Oei et al., 2012).
On the other hand, it has been reported that activity in the middle cingulate cortex is triggered during arousal, while activity in the posterior cingulate cortex appears to be negatively correlated with arousal (Stoléru, Fonteille, Cornélis, Joyal, and Moulier, 2012).
The white matter adjacent to the posterior cingulate region is a key target for deep brain stimulation to treat resistant depression, with its activity being pathologically elevated, leading to signs of relief in most patients (Mayberg et al., 2005).
Finally, increased activation of the cingulate cortex has been observed in males during erection and in females during orgasm (Schober & Pfaff, 2007).
Thus, synthesizing these findings suggests that this cortical region is involved in multiple features of sexual behavior, serving as a key relay structure between subcortical limbic structures and associated cortical areas.
3.3.1. Insula
As previously mentioned, the complexity of sexual behavior is primarily related to its conscious regulation. The insular cortex is a hub of the significant sexual network (Uddin, 2014; Wager & Barrett, 2004), processing sensory stimuli and relying on other cortical regions to facilitate attention and working memory. Several fMRI studies with sexual tasks have demonstrated the co-activation of the insula and ACC, indicating that these cortical regions are functionally coupled in some manner (Oei et al., 2012).
Similar to the cingulate cortex, the insula transmits integrated information to brainstem structures to regulate autonomic responses while relaying to motor-related areas to trigger appropriate responses to sexual stimuli (Both et al., 2008; Janssen, Everaerd, Spiering, and Janssen, 2000).
It has been found that the anterior insula is primarily active during the desire phase, while activity shifts to the posterior insula during the arousal phase (Georgiadis, 2015).
Given that midbrain dopaminergic projections simultaneously reach the insula and ACC as well as NAc, the functional coupling of these cortical regions is further enhanced when processing beneficial sexually relevant stimuli. An fMRI study conducted on patients with symptomatic epilepsy caused by insular lesions reported genital arousal disorders (Anzellotti et al., 2010).
According to Eickhoff et al. (2010), the body map will be computed in the posterior insula, indicating that it may play a role in processing tactile erotic stimuli. The location of the genitals in these maps remains to be determined (Eickhoff et al., 2010).
The representation of genitals in the human somatosensory cortex has recently been a topic of debate. Following tactile self-stimulation of the genitals, activation of the genital regions in the primary somatosensory cortex and secondary somatosensory cortex has been observed (Wise, Frangos, & Komisaruk, 2016).
The positions of male and female genitals in the so-called “genital cortex” have also been described in rats (Lenschow et al., 2016).
To gain a broader understanding of somatosensory representation, a systematic review by Cazala and colleagues is recommended (Cazala, Vienney, and Stoléru, 2015).
Moreover, the insular region has been used to make individuals aware of engorgement and congestion of erect organs during sexual arousal, with activation in the male insula being much larger (Rupp & Wallen, 2008).
4. Human Sexual Behavior from a Network Perspective
The past few decades have been characterized by a paradigm shift in the field of brain mapping, allowing for the study of the structure and function of the human brain not only at the level of individual regions but also from a network perspective.
Indeed, according to recent associative theories, the brain is composed of several isolated and parallel-distributed networks surrounding key and participating cortical hubs (Catani & Thiebaut de Schotten, 2012; Mesulam, 2000; Zappalà, Thiebaut de Schotten, & Eslinger, 2012), allowing for a good trade-off between the cost and efficiency of information transmission.
Neuroimaging studies indicate that human sexual responses involve multiple cortical and subcortical brain areas, exhibiting very similar activation patterns across different genders and sexual preferences.
Georgiadis and Kringelbach (2012) identified (a) a “sexual desire pattern” primarily involving the superior parietal lobule, temporoparietal area, NAc, OFC, ACC, amygdala, and hippocampus, and (b) a “sexual arousal pattern” involving the inferior parietal lobule, hypothalamus, insula, ventral premotor cortex, and middle cingulate cortex (Georgiadis & Kringelbach, 2012).
Abnormal activation patterns in the sexual desire network are associated with patients exhibiting sexual misconduct and hypersexuality (Kühn & Gallinat, 2016; Voon et al., 2014); on the other hand, brain activity induced by sexual cues shows a negative correlation with the severity of hypersexuality (Klucken, Wehrum-Osinsky, Schweckendiek, Kruse, and Stark, 2016).
In contrast, sexual interest involves the recruitment of a relatively different brain network from that of sexual demand. Changes in connectivity and gray matter within brain regions belonging to the sexual preference network have been observed in patients with psychogenic erectile dysfunction. In fact, different fMRI studies have shown altered connectivity between the insula and ventral premotor cortex, indicating that there may be abnormal inhibitory control. Recently, Zhao et al. compared the whole-brain network topology characterizing patients with psychogenic erectile dysfunction with healthy controls, showing a similar small-world organization (Zhao et al., 2015).
However, upon careful examination, the network topology of psychogenic patients shows an imbalance trade-off between local specialization and global integration, leading to reduced overall global integration of information transmission.
Behaviorally, the brain continuously acts to maintain a balance between networks that promote approach and those that promote avoidance. The prefrontal region seems to exhibit overactivity in patients with low sexual behavior, although the opposite result has been found in breast cancer survivors. In fact, Versace et al. (2013) provided these patients with erotic images, indicating reduced activity in the PFC and ACC, suggesting that chronic stressors may be related to the top-down regulation of the cortical and subcortical structures in the human sexual behavior network by the PFC (Versace et al., 2013).
The novel and rapidly evolving framework of network neuroscience has been reinforcing the notion that sexuality is a complex concept that relies on strict structural and functional interactions between brain regions that are spatially distant, working together to ensure the human sexual pleasure cycle.
5. Sexual Desire and Arousal
Sexual desire or libido is defined as a broad interest in sexual objects or experiences, while sexual arousal is both a subjective (i.e., feeling sexually excited) and physiological (i.e., genital vascular engorgement) term. While sex hormones play a crucial role in regulating sexual arousal, human sexual desire seems to be triggered by the reception/perception of sexual pheromones (Motofei, 2009), substances secreted by glands in the anus, urethra, breasts, and mouth (Motofei, 2009; Motofei and Rowland, 2005).
The potential mechanisms of universal arousal are complex and involve many brain circuits (Devidze, Lee, Zhou, and Pfaff, 2006).
There are five major neurochemical systems along the ventral and medial boundaries of the medulla and pons that contribute to the arousal of the forebrain, namely, systems signaled by norepinephrine, dopamine, serotonin, acetylcholine, and histamine, while the role of glutamate is less known.
Rostral and medial reticular neurons along the medulla and pons are important for regulating arousal in the central nervous system as they respond to pain, genital sensations, CO 2 levels in the blood, body temperature, and cardiovascular function changes. Other important axons descend from the paraventricular nucleus and the medial preoptic area of the hypothalamus, which influence arousal.
Ernst et al. proposed a neurobehavioral and multi-faceted model of sexual arousal mechanisms involving cognitive, emotional, motivational, and autonomic components (Ernst, Paine, and Hardin, 2006).
Brain regions associated with cognitive mechanisms of sexual arousal include the OFC and the “attention” network relay in the superior parietal lobule, while motivational components are believed to be stored in the tail of the ACC, related to the process of motor preparation; finally, autonomic mechanisms will involve the medullary parts of the hypothalamus, insula, and ACC (Ferretti et al., 2005).
In particular, neural pathways for sexual arousal induced by visual stimuli have been identified (Ferretti et al., 2005).
This circuit includes limbic (hypothalamus, hippocampus, and amygdala) and paralaminar regions (ACC, frontal lobe, and insula), associative cortex (inferior temporal and occipital cortex), and other subcortical and cortical sensory relays (thalamus and secondary somatosensory cortex – SII) (Calabrò & Bramanti, 2011; Ferretti et al., 2005).
It can be hypothesized that the autonomic and endocrine control of sexual behavior is mediated by the hypothalamus, while activation of the amygdala is related to the assessment process of evaluating erotic stimuli as sexual temptation. In fact, the amygdala participates in the emotional assessment of complex perceptual information related to visual processing of erotic stimuli (Sennwald et al., 2016).
The insula seems to be associated with the activation of the somatosensory processing pathway. Therefore, activation of this area, along with the thalamus and SII, may reflect participants’ awareness of their own behavioral responses (Calabrò & Bramanti, 2011).
6. Neurobiology of Sexual Function
In the past decade, there has been increasing research focus on the neurobiology of sexual function. This is due to a growing awareness of the detrimental effects of drugs on sexual behavior, the high prevalence of male sexual issues, and the tremendous success of phosphodiesterase inhibitors in treating erectile dysfunction. In this section, we briefly report the most important endocrine and neurotransmitter factors involved in sexual function (see Table 2 for a summary) (Calabrò & Bramanti, 2011).
Table 2 Summary of Major Neurotransmitters and Modulators Involved in Regulating Human Sexual Behavior
| Neurotransmitter and Modulator | Function in Regulating Human Sexual Behavior |
|---|---|
| Serotonin | Serotonin is mainly released by neurons in the raphe nuclei, acting on the smooth muscle of the vascular system of the genitals and other sexual organs, producing vasoconstriction and vasodilation. At the central level, it conversely has inhibitory effects on erectile function, lubrication, and sexual interest. |
| Dopamine | Dopamine in the striatum is important for the motor aspects of mating but not for sexual motivation. Depending on its concentration, dopamine in the MPOA can inhibit genital reflexes (low levels), promote parasympathetic-mediated erection and mating behavior (medium levels), and promote sympathetic-mediated ejaculation while inhibiting erection (high levels). |
| Norepinephrine | It stimulates penile erection through autonomic activation and can reverse sexual inhibition after sexual exhaustion, thus being used to treat erectile dysfunction and sexual arousal disorders. |
| Acetylcholine | It is associated with penile erection and has been shown to reverse erection and ejaculation difficulties caused by antidepressants. |
| Histamine | At the peripheral level, histamine leads to complete or partial erection by activating H2 and H3 receptors. At the central level, it conversely regulates sexual behavior and sexual desire. |
| Opioids | Opioids closely interact with hormones such as LH and testosterone, leading to sexual dysfunction. In particular, increased opioid activity, accompanied by decreased LH and testosterone levels, leads to decreased sexual desire, erectile dysfunction, and inability to achieve orgasm. |
| Sex Hormones |
Androgens play a crucial role in stimulating and maintaining male sexual function, being essential for the development, growth, and maintenance of erectile function of penile tissue. Estradiol is responsible for the behavioral development of male mammals, acting by increasing or decreasing male-typical behaviors. Furthermore, sex hormones seem to play an important role in sexual arousal by ensuring the integration of the brain between the body and autonomic system. Finally, prolactin provides sexual satisfaction to the body after sexual behavior, although high levels of prolactin in the blood may lead to erectile dysfunction and decreased sexual desire. |
6.1. Serotonin
Serotonin is a monoamine neurotransmitter that is widely present in the gastrointestinal tract of animals. Approximately 80%–90% of total serotonin in the human body is located in the enterochromaffin cells of the gut, used to regulate gut motility (Berger, Gray, & Roth, 2009).
The remaining serotonin is synthesized in serotoninergic neurons of the central nervous system, with various functions including regulating mood, appetite, sleep, muscle contraction, and some cognitive functions, including memory and learning. Neurons in the raphe nuclei are the primary source of 5-hydroxytryptamine (5-HT) release in the brain (Calabrò & Bramanti, 2011).
The axons of the tail neurons from the raphe nuclei terminate in the deep nuclei of the cerebellum, cerebellar cortex, and spinal cord. The axons of the head neurons from the raphe nuclei terminate in the thalamus, striatum, hypothalamus, NAc, neocortex, cingulate gyrus, hippocampus, and amygdala (Hornung, 2003).
Thus, the activation of this serotonin system affects most areas of the brain and seems to be related to sexual behavior (Hull, Muschamp, and Sato, 2004).
5-HT receptors are also located peripherally, and 5-HT acts on the smooth muscle of the vascular system of the genitals and other sexual organs, producing vasoconstriction and vasodilation effects.
In the CNS, 5-HT has inhibitory effects on sexual function (Croft, 2017).
Selective serotonin reuptake inhibitors (SSRIs) often impair ejaculation/orgasm function and frequently inhibit erectile function, lubrication, and sexual interest. Interestingly, experimental damage to the major source of spinal cord 5-HT, nPG1, suppresses urethrogenital reflexes (a model of orgasm) and reflexive erections and penile bending, confirming the potential inhibitory role of serotonin on sexual behavior.
5-HT receptors are highly heterogeneous and have been regrouped into seven different families. Although all 5-HT receptor subtypes are found postsynaptically and appear to mediate inhibitory effects on ejaculation, orgasm, and erection, only the 5-HT1A and 1B/D receptors are located presynaptically, mediating serotonin’s negative feedback release on their synapses (Giuliano & Clément, 2006).
Stimulation of 5-HT1A receptors in the MPOA can promote ejaculation, while systemic administration of 5-HT1A agonists can reverse sexual satiety. Thus, it has been proposed that the beneficial effects of 5-HT1A agonists may be due to the stimulation of inhibitory autoreceptors in the raphe nuclei, which would lower 5-HT levels. Otherwise, the facilitating effects of 5-HT1A agonists may be partially mediated by increases in extracellular DA in the MPOA (Hull, 2011).
Moreover, since 5-HT1A receptors have been found at the dorsal horn and the dorsal gray commissure, these receptors are likely to be involved in the spinal processing of sensory information transmitted to the brain while regulating the triggering of ejaculation (Hull et al., 2004; Meston & Frohlich, 2000).
6.2. Dopamine
The role of DA in human sexual behavior is not fully understood, and most of our knowledge comes from animal models (Hull et al., 2004).
Dopamine in the striatum inhibits the pathways that trigger cortical movement: this neurotransmitter is released during mating but not during exposure to receptive females before mating, indicating that ventral striatal DA (Dominguez & Hull, 2005) is important for the motor aspects of mating but not for sexual motivation. On the other hand, the role of DA in the MPOA is to promote male sexual behavior (Dominguez & Hull, 2005).
Considering that it has long been known that dopaminergic drugs clinically promote male sexual function, the fact that DA plays a key role in human sexual behavior is partially corroborated. In fact, it has been shown that the classic DA agonist apomorphine can effectively treat erectile dysfunction with minimal side effects. From a physiological perspective, a small increase in DA in the MPOA would relieve inhibition of genital reflexes via D2 receptor members; moderate increases promote parasympathetic-mediated erection and mating behavior; and large increases promote sympathetic-mediated ejaculation but inhibit erection (Dominguez, Gil, & Hull, 2006; van Furth, Wolterink, & Ree, 1995; Hull et al., 2004).
Interestingly, cocaine enhances dopaminergic activity during acute hypotheses by blocking presynaptic autoreceptors and enhancing dopamine release, and it is often regarded as an aphrodisiac that enhances sexual desire, sexual performance, and pleasure (Jones, Garris, and Wightman, 1995; Winton, 2006).
However, chronic cocaine users experience sexual dysfunction, such as suppressed sexual arousal, decreased libido, and delayed ejaculation, primarily due to damage to the dopaminergic system following persistent stimulation (Brown, Domier, and Rawson, 2005; Peugh and Belenko, 2001; Rawson, Washton, Domier, and Reiber, 2002).
However, the exact effects of acute and chronic cocaine use remain unclear, and they may be influenced by the context of drug use (Leigh, 1990).
Since it is known that cocaine has inhibitory effects on behavior, many people may be compelled to use this drug when expecting or seeking to engage in sexual intercourse (Kopetz, Reynolds, Hart, Kruglanski, and Lejuez, 2010).
6.3. Norepinephrine
As a stress hormone, norepinephrine (NE) is released from the adrenal medulla into the bloodstream, affecting bodily functions and controlling attention and response actions in the brain. In fact, along with epinephrine, NE is fundamental to the fight-or-flight response, directly increasing heart rate, triggering glucose release from energy stores, and increasing blood flow to skeletal muscles. Norepinephrine-containing neurons in the brain form a neurotransmission system that, when activated, impacts alertness, arousal, and the reward system. Anatomically, norepinephrine-containing neurons originate from the locus coeruleus and the lateral tegmental area, as observed in animal studies. The axons of locus coeruleus neurons act on adrenergic receptors in the following areas: amygdala, cingulate gyrus, insula, hippocampus, hypothalamus, neocortex, spinal cord, striatum, and thalamus. On the other hand, the axons of neurons from the lateral tegmental area act on adrenergic receptors in the hypothalamus (Calabrò & Bramanti, 2011).
Thus, NE release modulates different aspects of motivation through an “inverted U-shaped curve,” where optimal NE transmission supports optimal behavioral levels, but excessive transmission disrupts behavior by producing a generalized fear response. Norepinephrine activity plays a role in maintaining penile flaccidity and producing detumescence. A1 adrenergic receptors have been found in human penile tissue, and inhibition of α1 receptors leads to erection.
Research on the effects of drugs acting on NE receptors indicates that monoamines are important for male sexual function. As mentioned earlier, SSRIs produce numerous side effects, while novel antidepressants acting on norepinephrine transmission (i.e., venlafaxine, duloxetine, mirtazapine) have been found to produce fewer side effects (Calabrò, Manuli, Portaro, Naro, & Quattrini, 2018).
Considering that norepinephrine promotes human sexual behavior, balancing the negative effects of 5-HT on sexual behavior (by increasing brain norepinephrine levels) may prove that novel antidepressants acting on NE neurotransmission have lower incidence of sexual dysfunction compared to SSRIs (Behrens, Berg, Jbabdi, Rushworth, and Woolrich, 2007; Clayton, Haddad, Iluonakhamhe, Ponce Martinez, and Schuck, 2014; Hull et al., 2004; Johannessen Landmark, Henning, and Johannessen, 2016; Montejo, Montejo, and Navarro‐Cremades, 2015).
Interestingly, the administration of the α2 antagonist yohimbine stimulates penile erection through autonomic activation and can reverse sexual inhibition after male sexual exhaustion. Moreover, this drug is known to be used for treating erectile dysfunction and sexual arousal disorders (Bancroft, 2002a; Meston & Frohlich, 2000).
6.4. Acetylcholine
Along with vasoactive intestinal peptide, acetylcholine is related to penile erection, which occurs when smooth muscle in the corpus cavernosum of the penis relaxes, allowing more blood to flow into the penile tissue. Human penile corpus cavernosum is cholinergically innervated and contains cholinergic receptors, indicating that ACh has endogenous activity in penile tissue. Furthermore, cholinergic drugs such as carbachol have been shown to reverse erection and ejaculation difficulties caused by antidepressants (Bancroft, 2002a; Meston & Frohlich, 2000).
On the other hand, it is worth noting that yohimbine and carbachol do not seem to be useful drugs clinically, and we mention them here only for completeness.
6.5. Histamine
The role of histamine in regulating sexual behavior from the ventromedial nucleus of the hypothalamus (VMH) is well known from studies conducted on rats. H2 antagonists such as cimetidine and ranitidine have been shown to lead to decreased libido and erectile dysfunction, possibly due to reduced testosterone uptake (Calabrò & Bramanti, 2011).
Peripherally, histamine is related to penile vasodilation, as its injection into the corpus cavernosum produces complete or partial erection through the activation of H2 and H3 receptors (Zhou et al., 2007).
6.6. Opioids
Most knowledge about the role of opioids in the sexual response cycle comes from studies on the effects of agonists and antagonists of anesthetics and natural opioids (such as endorphins, enkephalins, and dynorphins) on humans and animals. In fact, the abuse of opioids has been shown to lead to decreased sexual desire, erectile dysfunction, and inability to achieve orgasm (Gulliford, 1998; Holloway, Cornil, and Balthazart, 2004; Pfaus and Gorzalka, 1987).
Withdrawal from opioid addiction in humans is characterized by increased morning frequency, spontaneous ejaculation, and slow recovery of sexual desire (Ouyang et al., 2012).
Although the mechanisms by which opioids affect sexual function are unclear, there is evidence that increased opioid activity lowers circulating hormone levels, such as LH and testosterone, leading to sexual dysfunction (Gudin, Laitman, and Nalamachu, 2015; Seyfried & Heister, 2012).
6.7. Sex Hormones
Sex hormones are crucial for the development of neural circuits and sex-specific behaviors. Male behaviors require testosterone and estrogen, but it remains unclear how these two hormonal pathways intersect. Circulating testosterone activates androgen receptors (AR) and is converted to estradiol in the brain via aromatase; this conversion, especially during critical periods of brain development, appears to be crucial for sexual behavior, differentiation, and orientation (Calabrò & Bramanti, 2011).
In the brain, testosterone binds to ARs but can be converted to dihydrotestosterone (DHT) through the 5-alpha-reductase pathway and bind to AR, or converted to estradiol and bind to estrogen receptors (ERs), which play a role in masculinization (increasing male-typical behaviors) and defeminization (reducing female-typical responses) in the behavioral development of male mammals. ARs are widely present in key brain and subcortical areas (MPOA, SNC, SDN, and INAH-3, also known as the nucleus of sexual orientation). A lack of estradiol in these key neural areas, influenced by genetics or reduced brain aromatase, may lead to the feminization of male sexual preferences (Bancroft, 2002a, 2002b).
Sex hormones seem to play an important role in sexual arousal by ensuring the integration of the brain between the body and autonomic system. In fact, they facilitate spinal reflexes to the brain level, which subsequently sensitizes genital stimulation by activating the autonomic nervous centers. Levels of sexual arousal are related to the natural occurrence of androgens and estrogens in a high-low ratio (high-low), which can induce central stimulant effects through gender differences in the sexual arousal and response sequence (Bancroft, 2002a; Lewis & Mills, 2004; Meston & Frohlich, 2000; Wu et al., 2009).
Androgens play a crucial role in stimulating and maintaining male sexual function; in particular, they are essential for the development, growth, and maintenance of erectile function of penile tissue. Increasing awareness has emerged that testosterone has profound effects on penile tissue involved in the mechanism of erection, as androgen deprivation leads to atrophy of penile tissue, changes in dorsal nerve structures, alterations in endothelial morphology, decreased smooth muscle content, and changes in extracellular matrix structure. Moreover, testosterone is involved in clitoral engorgement and genital lubrication (Nappi et al., 2003).
It is believed that testosterone levels required for sexual interest and activity in adult males are below the normal levels of testosterone in circulating males. Therefore, the variability of testosterone levels above this threshold or exogenously induced changes in testosterone above this level are not expected to affect sexual interest or behavior. On the other hand, it is clear that decreased testosterone is associated with decreased sexual desire in both males and females (Andersen & Tufik, 2006).
Lower testosterone levels in females due to pituitary dysfunction and premature ovarian failure are associated with decreased sexual desire, typical hypoactive sexual desire disorder, and female sexual arousal disorder (Kingsberg & Rezaee, 2013).
Testosterone therapy is currently used to treat hypoactive sexual desire disorder (Abdallah & Simon, 2007).
The physiological basis of sexual desire seems to depend on the action of androgens on the paraventricular nucleus of the hypothalamus, which is the integration center between the central and peripheral autonomic nervous systems, although it projects to many important brain regions controlling penile erection (Morales et al., 2016, 2004, 2009).
On the other hand, estrogens may have little direct effect on sexual desire, as relatively high levels of exogenous estrogens in males partially suppress the sexual desire of sexual offenders.
In contrast, prolactin (PRL) provides sexual satisfaction to the body after sexual behavior. Sexual arousal and sexual stimulation themselves do not significantly alter prolactin levels, but changing post-orgasm levels may be important for explaining refractory and decreased sexual desire. Recently, it has been hypothesized that PRL may represent a negative feedback mechanism, as this hormone may alter the activity of dopaminergic neurons in the CNS, particularly in the nigrostriatal and mesolimbic cortical systems and MPOA, controlling different aspects of sexual behavior. Interestingly, a case study indicated that males with multiple orgasms significantly lack orgasm-induced PRL secretion, paralleling the extremely short refractory period.
Finally, 2003; Krüger, Haake, Hartmann, Schedlowski, and Exton, 2002).
7. Author Comments, Clinical Implications, and Conclusion
In this review, we aim to describe the neuroanatomical and physiological basis of human sexual behavior, illustrating how the brain initiates and maintains sexual arousal and desire, ultimately leading to the orgasm stage. Despite the increasing importance of knowledge regarding human sexual behavior, considering its social and personal implications, it remains unclear whether this topic is adequately addressed or completely overlooked in clinical practice due to a lack of education and training on human sexual behavior in medical schools and throughout the life course. Nevertheless, understanding the neural correlates and functions of human sexual behavior is fundamental for better patient management, especially for those with neurological diseases (Calabrò et al., 2014; Calabrò, Gervasi, and Bramanti, 2011; Calabrò, Marino, and Bramanti, 2011).
In fact, injuries to the brain and spinal cord, as well as peripheral diseases, may frequently affect sexual function, leading to a decline in quality of life. Many times, physicians consider sexual issues less important than the harm or disease that brings patients to the medical team. The quality of interpersonal relationships, particularly the quality of sexual relationships, has a significant impact on patients’ self-esteem and support networks. Various physical, psychological, and emotional changes that may occur after catastrophic injuries or due to congenital disabilities or chronic illnesses must be addressed not only in the context of the patient but also within the patient’s support system.
Calabrò RS, Cacciola A, Bruschetta D, Milardi D, Quattrini F, Sciarrone F, la Rosa G, Bramanti P, Anastasi G. Neuroanatomy and function of human sexual behavior: A neglected or unknown issue? Brain Behav. 2019 Dec;9(12):e01389. doi: 10.1002/brb3.1389. Epub 2019 Sep 30. PMID: 31568703; PMCID: PMC6908863.