
Pulmonary Valve Anatomy

Anatomy of the Right Ventricular Outflow Tract to the Pulmonary Artery
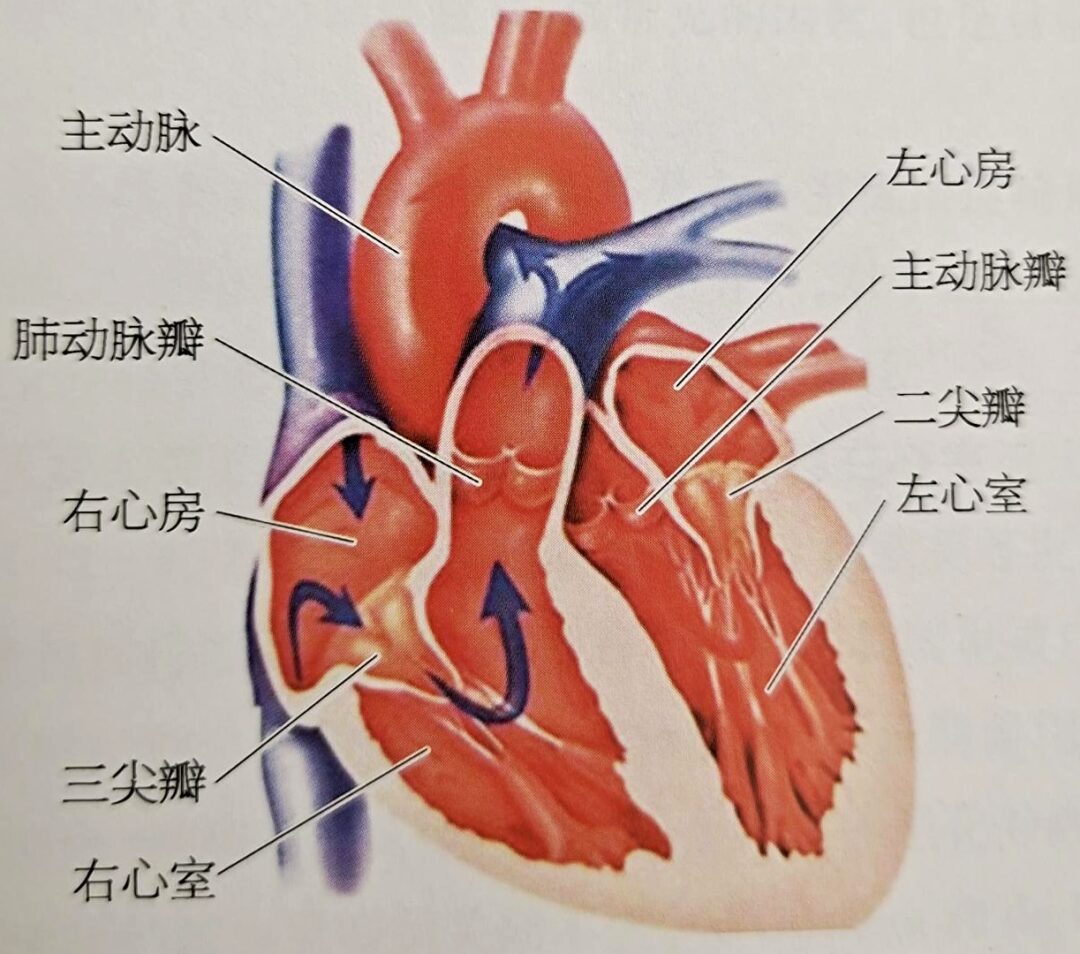
Surrounding the pulmonary artery orifice is the pulmonary valve annulus, which is attached to three crescent-shaped leaflets, known as the pulmonary valve. When the ventricle contracts, blood flows through the pulmonary valve into the pulmonary artery; during ventricular relaxation, the valve closes, preventing blood from flowing back into the ventricle. The three leaflets of the pulmonary valve, the cusps, and the annulus are relatively weak, and the annulus connects to the muscle of the right ventricular outflow tract (RVOT), without a direct fibrous continuity with the tricuspid valve. The three leaflets can be classified as the left, right, and anterior leaflets. The left leaflet is continuous with the septal band of the conus, while the right leaflet is continuous with the wall of the outflow tract. The inner half of the left and right leaflets is in contact with the aortic wall, and the junction between the left and right pulmonary valve leaflets corresponds to that of the aortic left and right leaflets, although these junctions do not connect at the same point, with the junction of the pulmonary valve being slightly higher. The anterior leaflet of the pulmonary valve is attached to the free wall of the right ventricle. Above the pulmonary valve is the pulmonary artery trunk. The pulmonary artery trunk is located within the pericardium and is a short, thick arterial trunk that originates from the right ventricular outflow tract, slanting left and posterior above the ascending aorta, and bifurcating into the left and right pulmonary arteries below the aortic arch.

What Are the Causes of Pulmonary Valve Regurgitation?

(1) Congenital pulmonary valve malformations:
(2) Marfan syndrome:
(3) Cardiac tumors:
(4) Pulmonary valve annulus dilation:
(5) Iatrogenic causes:

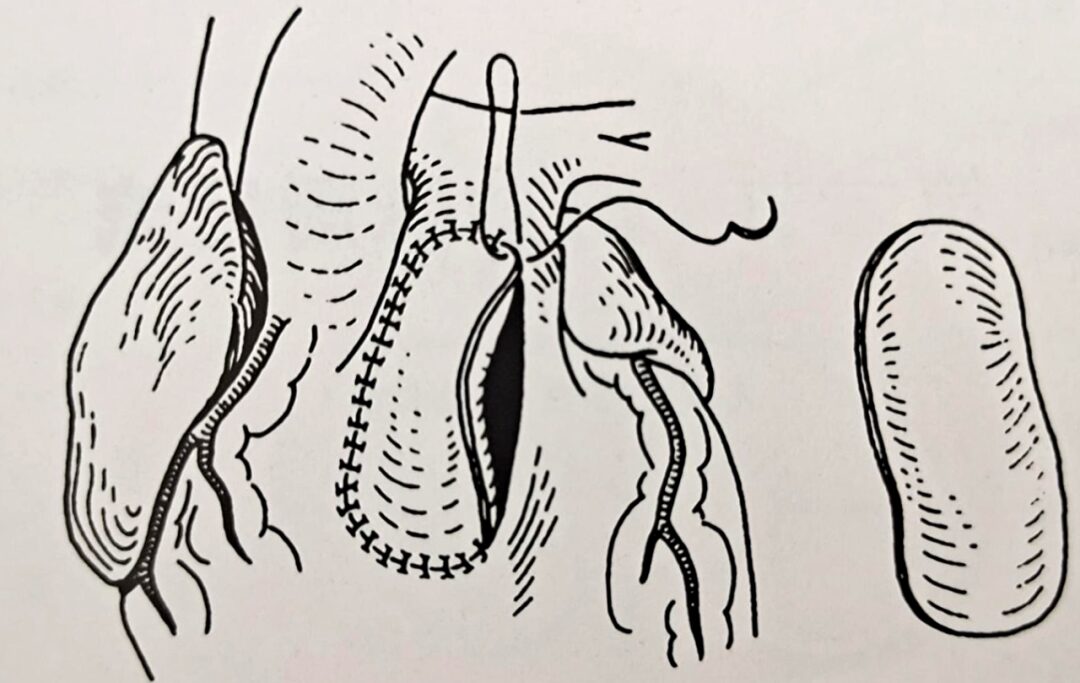
Patch for Transpulmonary Valve

Pulmonary Valve Regurgitation Pathophysiology


Choosing the Timing of Intervention



1. Indications
(1) Moderate to severe pulmonary valve regurgitation following surgical correction of congenital heart disease with RVOT stenosis.
(2) Patients with symptoms related to right ventricular outflow tract stenosis, including decreased exercise tolerance or right heart failure; or asymptomatic patients with any of the following: ① Moderate or greater functional tricuspid regurgitation; ② Cardiac MRI showing RVEDVI ≥130ml/m²; ③ Cardiac MRI showing right ventricular ejection fraction <45%, QRS duration ≥160ms; ④ Persistent atrial or ventricular arrhythmias.
(3) Anatomically suitable for PPVI.
(4) Age ≥10 years or weight ≥25kg.
2. Contraindications
(1) Pulmonary hypertension [mean pressure ≥25mmHg (1mmHg=0.133kPa)].
(2) Severe pulmonary artery (PA) or branch stenosis.
(3) Anatomical assessment unsuitable, including inability to deliver the valve through vascular access or inability to place the valve in the RVOT-PA, or preoperative evaluation suggests that the valve stent may compress the coronary artery.
(4) Presence of contraindications for catheterization.


END
Source: Long Yuliang, Zhang Xiaochun, Pan Wenzhi. “Transcatheter Heart Valve Procedures”. Shanghai Scientific and Technical Publishers
1.Shimazaki Y, Blackaone EH, Kirklin JW. The natural history of isolated congenital pulmonary valve incompetence: surgical, implications[J]. Thorac Cardiovase Surg, 1984, 32:257-259.
2.McElhinney DB. Hennesen JT. The Melody valve and Ensemble delivery system for transcatheter pulmonary valve replacement[J]. Ann NY Acad Sci, 2013, 1291:77-85.
3.Murphy JG. Gersh BJ, Mair DD, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot[J]. N Engl J Med, 1993, 329:593-599.
4.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Candiology/American Heart Association Task Force on Practice Guidelines [J]. J Am Coll Cardiol, 2008, 52:e143-263.
5.d’Udeiem Y, Ovaert C, Grandjen F, et al. Tetralogy of Fallot: transannular and right ventricular patching equally affect late functional status[J]. Circulation, 2000, 102(19 Suppl 3):Ⅲ116-Ⅲ122.
6.de Ruijter FT, Weenink I, Hitchcock FJ, et al. Right ventricular dysfunction and pulmonary valve replacement after correction of tetralogy of Fallot[J]. Ann Thorac Surg, 2002, 73(6):1794-1800.
7.Hui Wei, Sun Bin, Huang Guoying, et al. Evaluation of the mid- and long-term efficacy of 109 cases of tetralogy of Fallot after surgery[J], Chinese Journal of Ultrasound Medicine, 2001 (4): 33-35.
8.Jiang Rui, Yan Jun, Li Shoujun, et al. Clinical analysis of 178 cases of tetralogy of Fallot repair[J]. Clinical Journal of Cardiovascular Diseases, 2011 (9): 702-704.
9.Ji Guangyu, Xu Zhiyun, Wang Zhongnong, et al. Long-term efficacy of surgical treatment for adult tetralogy of Fallot[J]. Chinese Journal of Thoracic and Cardiovascular Surgery, 2010 (4): 287-291.
10.Xie Zhaofeng, Zhang Zhiwei, Xu Yanmei, et al. Long-term efficacy and complications after repair of tetralogy of Fallot[J]. Clinical Journal of Cardiovascular Diseases, 2013, 29 (8): 618-620.
11.Long Yuliang, Pan Wenzhi, Zhan Zhi, et al. Analysis of pulmonary valve function in patients after surgical repair of tetralogy of Fallot[J]. Chinese Journal of Cardiovascular Diseases, 2017, 45 (8): 722-725.
12.Chaturvedi Re, Redington AN. Pulmonary regurgitation in congenital heart disease[J]. Heart, 2007, 93:880-889.
13.Nollert GD, Dabritz SH, Schmoeckel M, et al. Risk factors for sudden death after repair of tetralogy of Fallot[J]. Ann Thorac Surg, 2003, 76:1901-1905.
14.Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study[J]. Lancet, 2000, 356:975-91.
15.Harrison DA. Harris L. Siu Sc. at al. Sustained ventricular in adult patients late after repair of tetralogy of Fallot[J]. J Am Coll Cardiol, 1997, 30:1368-1373.
16.Gatzoulis MA. Till JA, Somerville J, et al. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death[J]. Circulation, 1995, 92:231-237.
17. Ferraz Caralcanti PE, Sa MP, Santos CA, et al. Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta-analysis and meta-regression of 3,118 patients from 48 studies[J]. Journal of the American College of Cardiology, 2013, 62(23):2227-2243.

● Observational Analysis of Echocardiography in Patients with Atrial Fibrillation
● The Relationship Between the Left Atrial Appendage and Atrial Fibrillation
● What Is Mitral Regurgitation? You Will Understand After Reading!
● Understanding the Mitral Valve!
