J. Am. Chem. Soc. | Achieving Enantiomer Purification through Directed Evolution of Crystal Stability and Non-Equilibrium Crystallization

Today, I would like to share an article published in J. Am. Chem. Soc. titled “Achieving Enantiomer Purification through Directed Evolution of Crystal Stability and Non-Equilibrium Crystallization.” The corresponding author of this paper is Professor Noorduin from the Van ‘t Hoff Institute for Molecular Sciences at the University of Amsterdam.
Crystallization is a simple, direct, and commonly used method for separating chiral molecules and isolating their pure enantiomers. However, there is a fundamental requirement for chiral purification through crystallization: the enantiomers must spontaneously separate into individual enantiomerically pure crystals (such as racemic cluster crystals) (Figure 1a). Unfortunately, this self-separation behavior is rare and unpredictable: the vast majority of enantiomer mixtures crystallize together into thermodynamically more favorable racemic compounds (90-95%), complicating direct crystallization for chiral separation. Overcoming these fundamental thermodynamic limitations not only opens up new systematic and general methods for discovering and utilizing cluster crystals but also allows for the development of new strategies for directly splitting or even deracemizing racemic compounds.
The authors propose that these thermodynamic limitations can be overcome by utilizing non-equilibrium conditions to kinetically promote the formation of cluster crystals. Under such conditions, the rates of nucleation and crystal growth, rather than thermodynamic stability, may determine which crystal phase predominates, similar to polymorphic phenomena. This suggests the possibility of utilizing non-equilibrium conditions to promote the formation of cluster crystals at the expense of thermodynamically stable racemic compounds. Previous reports have supported this view—under non-equilibrium conditions, through grinding crystals or applying significant temperature gradients during cooling crystallization, racemic compounds can be converted into enantiomerically pure crystals. Although these cases are promising, it remains unclear whether these situations are merely incidental reports for systems with specific characteristics or if there are general guidelines that can be extended to separate enantiomers through crystallization systems.
When the energy difference ΔGΦ between two phases is small, it is feasible to convert from the racemic crystal phase to its enantiomerically pure crystal phase (Figure 1b). In fact, for polymorphic transitions, it is generally accepted that when ΔGΦ<0.5 kcal mol−1(2.1 kJ mol−1), the thermodynamically stable phase may convert to the kinetically stable phase. Previous analyses have been conducted on ΔGΦ for many chiral compounds, which have been used to identify indicators of thermodynamically stable racemic cluster crystals. However, these early analyses only involved sporadic reports, and the molecules studied had no structural similarity. Currently, there is still a lack of systematic analysis of ΔGΦ between racemic crystals and their enantiomerically pure substances for structurally related compounds. Such analyses could not only systematically discover kinetically stable crystal structures but also provide relevant guidance for experimental design—by systematically utilizing non-equilibrium conditions to deracemize racemic compounds into (kinetically stable) cluster crystal counterparts (Figure 1c).
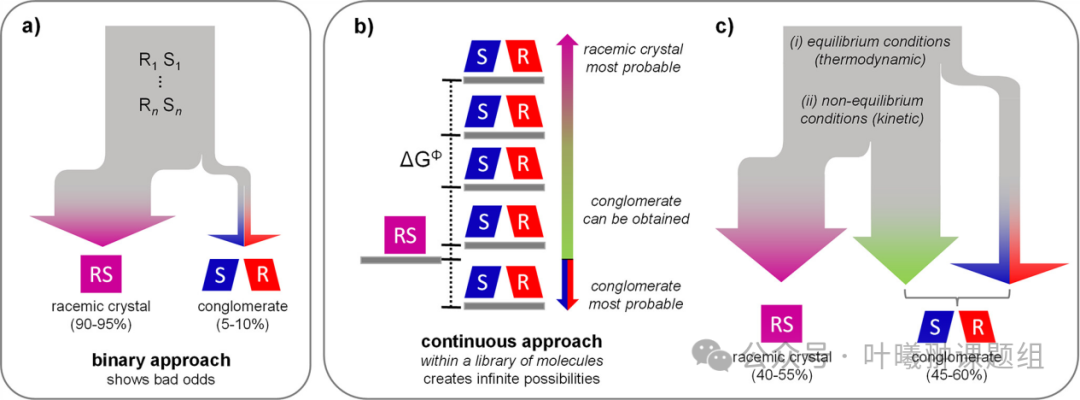
Figure 1: Stability of racemic compounds and cluster crystals under thermodynamic and kinetic conditions. (a) Current binary classification: thermodynamics determines whether chiral molecules form racemic crystals (90-95%) or cluster crystals (5-10%); (b) Derivatives with common chiral centers can construct molecular libraries for screening the continuous energy difference (ΔGΦ) between racemic and enantiomerically pure crystal forms; (c) For small positive ΔGΦ values, non-equilibrium conditions can (kinetically) stabilize the enantiomerically pure crystals of thermodynamically stable racemic compounds, with an expected 45-60% of chiral molecules obtainable in the form of kinetic or thermodynamic cluster crystals.
Based on the insights above, this study systematically analyzed the ΔGΦ between racemates and their enantiomerically pure counterparts in two libraries of biologically relevant target molecules with different chemical modifications. The study found that these molecular libraries exhibited a wide and continuous distribution of ΔGΦ, with molecules of similar chemical modifications showing clustered distribution characteristics. Similar to the directed evolution strategies in the field of catalysis, the authors foresee that systematic screening for chemical modifications with the lowest ΔGΦ can guide the synthesis of next-generation molecules (Figure 2). This evolutionary approach is expected to efficiently identify metastable enantiomerically pure crystal phases, thereby separating target enantiomers under non-equilibrium conditions. Analysis of over a hundred chiral molecules in the literature indicates that the authors found universality—over 50% of chiral molecules tend to separate in enantiomerically pure form under non-equilibrium conditions (Figure 1c).
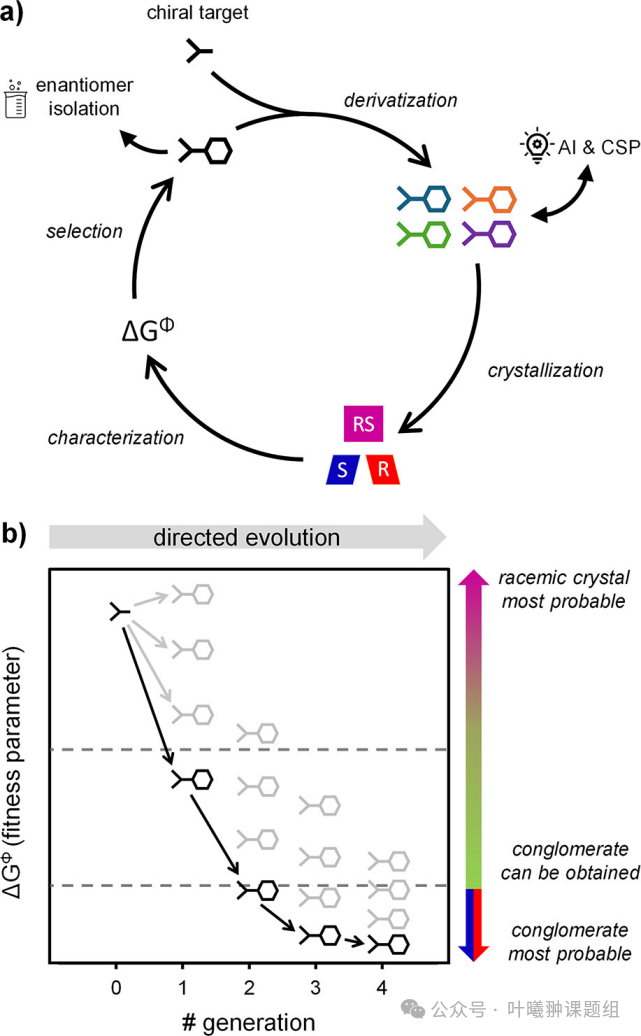
Figure 2: Discovery of (kinetic) cluster crystals and directed evolution strategies for molecular library design. (a) Development of target chiral (kinetic) cluster crystals through iterative cycles of chemical derivatization, crystallization, characterization, and screening. ΔGΦ serves as a fitness parameter for selecting the next generation of input molecules, with artificial intelligence (AI) or crystal structure prediction (CSP) assisting in guiding derivative selection and integrating previous characterization results. (b) Iterative evolution of target derivatives systematically trends towards lower ΔGΦ values. Due to diminishing marginal returns with each iteration, fitness parameters typically follow a power law or exponential decay to reach a steady state. This directed evolution strategy can rapidly discover (meta)stable enantiomerically pure crystal phases for separating target enantiomers.
The authors conducted a systematic study of the ΔGΦ between enantiomerically pure crystal forms and racemic crystal forms for a set of structurally similar chiral molecules. They selected phenylalanine amide Schiff base 1 as the chiral core—this amino acid derivative is a structural unit of various pharmaceutical compounds and has previously achieved deracemization through the cluster crystal form of 1a. These Schiff base derivatives can be directly synthesized from aldehyde compounds, thus constructing a molecular library (Figure 3a). Based on this method, a library of 19 derivatives (including enantiomerically pure and racemic crystal forms) with the same chiral core was obtained (Figure 3b).
The authors determined the ΔGΦ between each enantiomerically pure crystal form and racemic crystal form in this molecular library. The melting points and melting heats of the two crystal forms were obtained through DSC, and ΔGΦ was calculated accordingly. Figure 3c shows the cumulative probability density distribution of ΔGΦ. Although the size of molecular library 1 is small, its ΔGΦ has shown a wide distribution from close to 0 to 1.5 kcal/mol. The stable cluster crystal 1a and its derivatives 1b and 1c (which, although identified as racemic compounds, have previously achieved deracemization) all have ΔGΦ values concentrated around ≈0.1 kcal/mol. This clustering phenomenon is expected: thermodynamically stable racemic compounds with lower ΔGΦ values may be suitable for conversion into kinetically stable cluster crystals.
To further explore the predictive potential of ΔGΦ, the authors selected compound 1d for deracemization experiments (Figure 3c). In the experiment, a solution of racemate 1d was prepared, and after initiating the racemization reaction under alkaline conditions, enantiomerically pure (R)-1d seeds were added. After 3 hours of grinding treatment, it was observed that (RS)-1d was completely converted to enantiomerically pure (R)-1d. This successful deracemization experiment confirms that this compound formed a (meta)stable aggregate state, and ΔGΦ can indeed be used to predict the conversion to enantiomerically pure crystals.
Compounds 1a-d not only exhibit similar ΔGΦ values, but their crystal structures are also highly similar—all crystal structures exhibit the same hydrogen bonding pattern. From the molecular structure, it is possible to predict potential crystal structures and further predict the corresponding ΔGΦ. The data from this study indicate that ΔGΦ can even be directly predicted from the molecular structure (without considering intermediate factors of crystal structure). This correlation will aid in the systematic design of molecular libraries with low ΔGΦ.
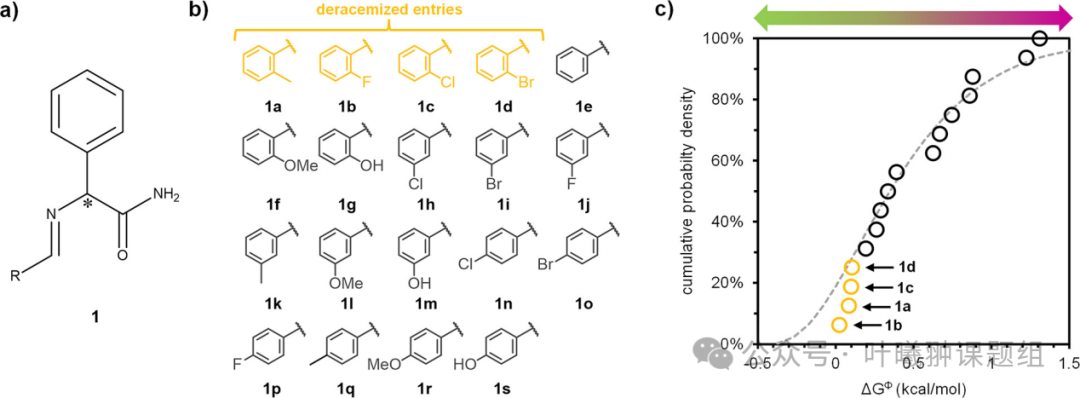
Figure 3: Analysis of the free energy difference (ΔGΦ) between racemates (RS) and enantiomerically pure (R) crystal forms in a molecular library with a common chiral center. (a) Schiff base derivatives of phenylalanine amide (1), *indicating the position of the chiral center; (b) Synthesis molecular library of compound 1; (c) Cumulative probability distribution of free energy differences showing a wide variation in ΔGΦ (the gray dashed line is the gamma distribution fitted based on literature data from Figure 5b). Low ΔGΦ compounds 1a-d (highlighted in yellow) have successfully achieved deracemization.
Drawing on the directed evolution strategies in the field of catalysis, the authors propose that by iteratively screening for low ΔGΦ molecules, this can systematically guide the design of chiral center modification structures to obtain compounds with low ΔGΦ characteristics. Since ΔGΦ has continuous variation characteristics (unlike the traditional binary classification of cluster crystals/racemic compounds), it can serve as an ideal fitness parameter in the directed evolution process.
To evaluate the potential of this molecular design evolution method, a molecular library was synthesized based on the chiral core of piperaquine (2) (Figure 4a). Piperaquine (2h) is a racemic drug for anti-parasitic treatment, and there is widespread interest in separating its bioactive enantiomer (R)-2h. To systematically study the correlation between chemical structure and ΔGΦ, the authors prepared 25 derivatives and categorized them into four types of modifications (Figure 4b): alkyl (2a-e), carbocyclic (2f-h), arylalkyl (2i-o), halogenated and other aromatic substituents (2p-v), and three unclassified derivatives (2w-y).
The authors determined and plotted the cumulative probability density distribution of ΔGΦ (Figure 4c). With a few exceptions, the compounds in the molecular library clustered along the ΔGΦ values according to the preset modification categories, which provides potential for the evolutionary strategy of molecular library design (Figure 2). Specifically, when only four compounds (one from each modification category) were used as the first generation starting samples, the alkyl derivatives were identified as the most promising candidates due to their lowest ΔGΦ values. Subsequently, when a second generation sample containing four additional alkyl derivatives was prepared, stable cluster crystal 2a was obtained. This demonstrates that compared to preparing 25 random derivatives, only two generations of screening (with a total sample size of less than one-third, 8 instead of 25) were needed to discover cluster crystals and low ΔGΦ compounds, fully showcasing the potential of directed evolution strategies in molecular library design.
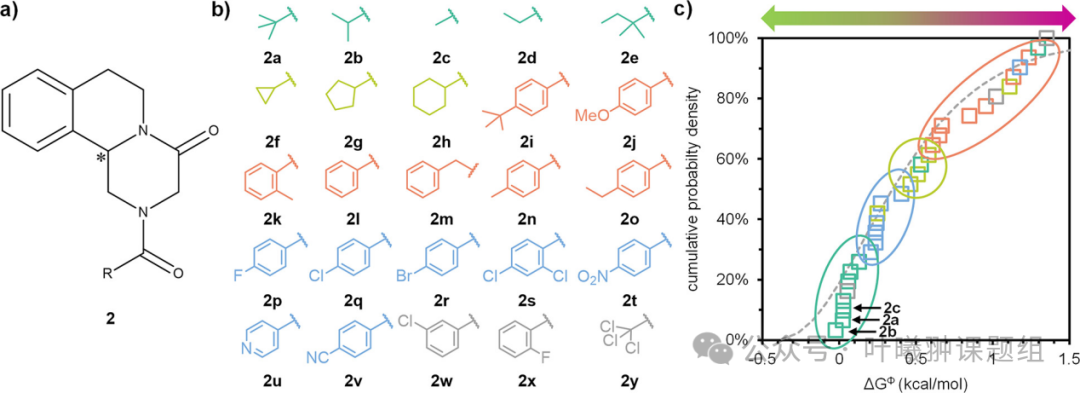
Figure 4: Potential of directed evolution molecular library design. (a) Derivatives of piperaquine 2, *indicating the position of the chiral center; (b) Synthesis molecular library of compound 2, categorized by alkyl (dark green), carbocyclic (light green), arylalkyl (orange), halogenated and other aromatic substituents (light blue), and unclassified (gray); (c) Cumulative probability distribution of free energy differences (the gray dashed line is the gamma distribution fitted based on literature data from Figure 5b). Derivatives exhibit clustering characteristics according to chemical classification (colored ellipses), which provides potential for directed evolution molecular library design.
The clustering distribution of chemical categories not only provides potential for directed evolution design of molecular libraries but also allows racemic compounds to fall within the distribution range of ΔGΦ, making these compounds suitable for separating enantiomerically pure crystals through kinetically stable cluster crystals. To verify this idea, the authors explored whether racemic compounds 2b and 2c (which are in the same low ΔGΦ region as the known stable cluster crystal 2a) could serve as enantiomerically pure crystal separators. To this end, the authors prepared supersaturated racemic solutions of 2b and 2c, added (R)-2b and (R)-2c seeds, and ultimately obtained the target enantiomers with good yield and high enantiomeric purity (>95% ee).
The chemical core structures of the two molecular libraries differ significantly: compound 1 has a small molecular weight, high flexibility, and can form hydrogen bonds, while compound 2 has a large molecular weight, high rigidity, and cannot form hydrogen bonds. To understand how these differences affect the distribution of ΔGΦ, probability histograms of ΔGΦ for 1 and 2 were plotted (Figure 5a). The comparison shows that despite the different molecular structures, the distributions of ΔGΦ for both are remarkably similar. Furthermore, in both molecular libraries, when ΔGΦ<0.2 kcal/mol, it is possible to directly separate enantiomerically pure crystals from racemic compounds under near-equilibrium conditions (Figure 5a). These commonalities raise two key questions: first, is this pattern universal? Second, can the threshold of ΔGΦ for converting racemic compounds into kinetically cluster crystal states be further broken through by utilizing non-equilibrium conditions?
To explore the universality of this pattern, the authors collected thermodynamic data from over a hundred reported chiral racemic compounds. This literature molecular library is diverse: it includes both salts and multi-chiral center molecules, covering functional groups with various heteroatoms such as sulfur, nitrogen, and oxygen. More importantly, unlike the two molecular libraries in this study, most compounds in the literature dataset lack structural correlation, thus serving as a representative reference for assessing universality. The authors found that the literature data conforms to a gamma distribution (Figure 5b), and the Kolmogorov-Smirnov test indicates that molecular libraries 1 and 2 also follow this distribution (Figures 3c and 4c). This consistency in distribution suggests that the patterns observed in the two molecular libraries can be generalized to a large system of structurally diverse chiral organic molecules.
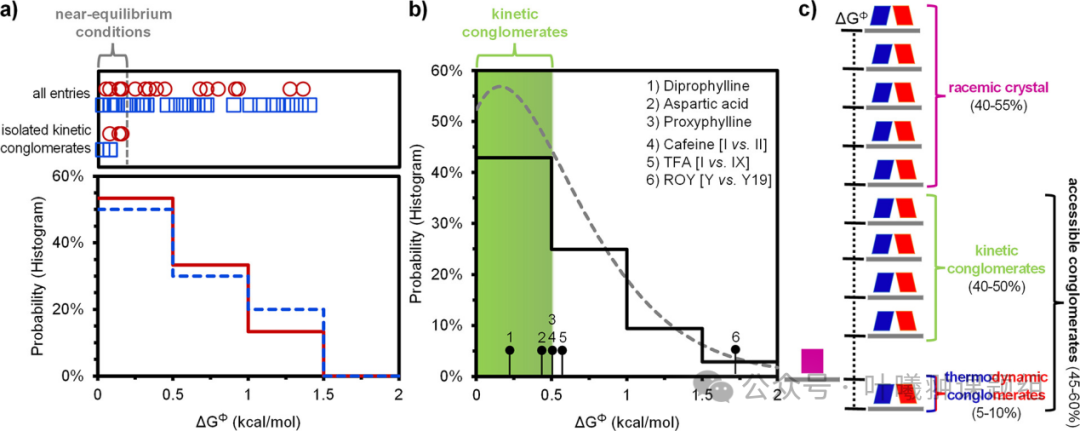
Figure 5: Universality of patterns and potential for separating enantiomerically pure crystals under non-equilibrium conditions. (a) Despite chemical differences, the ΔGΦ probability distributions of molecular libraries 1 (red solid line histogram) and 2 (blue dashed line histogram) are highly similar. Compounds separated in the form of kinetically cluster crystals under near-equilibrium conditions all fall within the ΔGΦ<0.2 kcal/mol range; (b) Probability histogram of ΔGΦ for over a hundred non-correlated chiral molecules in the literature (black solid line). The histogram shapes of the three datasets are similar and all conform to the same gamma distribution (gray dashed line), confirming the universality of this pattern. The ΔGΦ<0.5 kcal/mol range (green shading) includes kinetically cluster crystals (1-3) and unstable polymorphs (4,5), suggesting that approximately 40-50% of racemic compounds can be obtained as enantiomerically pure crystals under near-equilibrium or non-equilibrium conditions; (c) Predicted distribution shows the proportional relationship between thermodynamic cluster crystals, kinetic cluster crystals, and racemic crystals.
Based on the above analysis, the authors further evaluated the universal potential of kinetically stable enantiomerically pure crystals under non-equilibrium conditions. They selected the three chiral molecules: theobromine, aspartic acid, and procaine as research subjects—whose racemic compounds have previously achieved kinetic conversion to enantiomerically pure crystals, with ΔGΦ values calculated to be 0.24, 0.45, and 0.48 kcal/mol, respectively, and marked for comparison in the energy difference distribution diagram in Figure 5b. The study found that all three conversions adopted crystallization conditions favorable for the kinetic phase, indicating that non-equilibrium conditions are a key factor in achieving deracemization.
The ΔGΦ values of these compounds are close to thermal energy kBT (0.6 kcal/mol), indicating that transitions between crystal phases with such energy differences (ΔGΦ≤ 0.5 kcal/mol) are kinetically feasible. This view is consistent with observations outside of chiral crystallization—when the energy difference is below 0.5 kcal/mol, polymorphic transition phenomena are frequently reported. Typical examples include caffeine (ΔGΦ=0.5 kcal/mol) and tofenamic acid (TFA, ΔGΦ=0.55 kcal/mol) (Figure 5b). Some reported conversions even have larger energy differences, such as the typical polymorphic system ROY with ΔGΦ as high as 1.7 kcal/mol. Therefore, the authors estimate that 40-50% of thermodynamically stable racemic compounds (ΔGΦ≤0.5 kcal/mol) may obtain enantiomerically pure crystals through kinetic pathways under near-equilibrium or non-equilibrium conditions (Figure 5b). Additionally, another 5-10% of chiral compounds crystallize as stable cluster crystals. In summary, it is predicted that through crystallization processes under equilibrium or non-equilibrium conditions, 45-60% of chiral compounds can achieve the separation of target enantiomers (Figure 5c).
By systematically studying the energy differences between racemates and enantiomerically pure crystal forms of structurally related molecules, the authors elucidate for the first time: by combining directed evolution and combinatorial chemistry strategies, it is possible to discover enantiomerically pure crystal phases that can be achieved through kinetically stable metastable states, thereby achieving the separation of target chiral enantiomers. Traditional understanding, constrained by thermodynamic limitations, has believed that only 5-10% of chiral molecules can be obtained in the form of enantiomerically pure crystals; however, this study confirms that through non-equilibrium crystallization strategies, at least 50-65% of chiral molecules can achieve this goal.
These findings can be directly applied to guide the crystallizable separation of chiral compounds. Although a single atomic change can significantly affect crystal phase stability, it has also been observed that similar derivatives in the molecular library form clustered distributions, laying the foundation for systematic design of molecular libraries. Specifically, the authors envision using iterative strategies to autonomously construct chemical molecular libraries in automated laboratories—by rapidly determining ΔGΦ as a diagnostic indicator, it can guide the design of new molecules and efficiently screen suitable target molecules for separation or deracemization. Similar to the directed evolution strategies in the field of catalysis, the authors suggest first synthesizing a small molecular library with high diversity, ranking them using ΔGΦ as a fitness parameter, and then selecting the optimal molecules as starting points for the synthesis of the next generation of molecules. This evolutionary strategy avoids merely screening unfavorable regions of high ΔGΦ, instead rapidly guiding towards advantageous regions of low ΔGΦ in a few iterative cycles. It is foreseeable that combining crystal structure prediction (CSP) methods will further enhance the accuracy of design decisions.
For compounds with low ΔGΦ values, this study indicates that it is possible to successfully achieve the separation of enantiomerically pure crystals from racemic mixtures under near-equilibrium conditions. The next key breakthrough lies in the systematic development of crystallization conditions that are far from equilibrium—under such conditions, the crystallization rate (rather than mere thermodynamic stability) will become the key factor determining the dominant crystal phase, such as enabling the growth rate of target kinetic cluster crystals to surpass that of non-target racemic compounds. Additionally, specific non-equilibrium conditions can be utilized to suppress the nucleation and growth rates of stable racemic compound crystals, thereby achieving selective separation of enantiomerically pure crystals. It is particularly important to emphasize that the crystallization process provides a wealth of tunable parameters to achieve ideal nucleation and growth kinetics, including solvent selection, spatial constraints such as microdroplet confinement, (chiral) additive regulation, temperature gradient settings, and mechanochemical methods. In fact, mechanical grinding and temperature gradient techniques have been successfully applied to achieve solid-state deracemization, indicating that constructing non-equilibrium conditions can effectively weaken the stability of racemic compounds while simultaneously converting racemic (or partially enriched) solids into target enantiomers. With the rapid development of machine learning technologies and automated laboratories in the synthesis of chiral molecules, assessing the potential of key intermediates for separation or deracemization will undoubtedly become an indispensable part of the synthesis process for enantiomerically pure molecules.
Text: Zhang Xi
Reviewed by: Ye Xichong
References:DOI: 10.1021/jacs.5c00569
https://doi.org/10.1021/jacs.5c00569