 Decarboxylative Cross-Coupling Enabled by Fe and Ni Metallaphotoredox CatalysisJ. Am. Chem. Soc. 2024, 146, 29551−29559
Decarboxylative Cross-Coupling Enabled by Fe and Ni Metallaphotoredox CatalysisJ. Am. Chem. Soc. 2024, 146, 29551−29559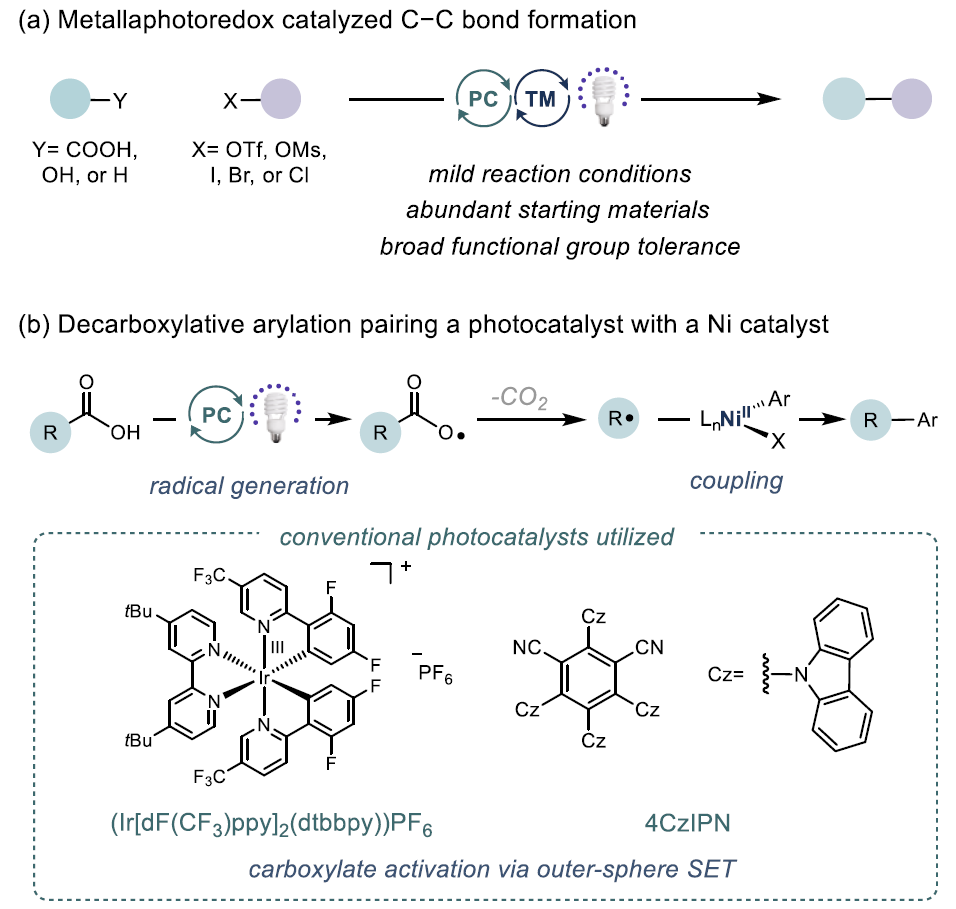
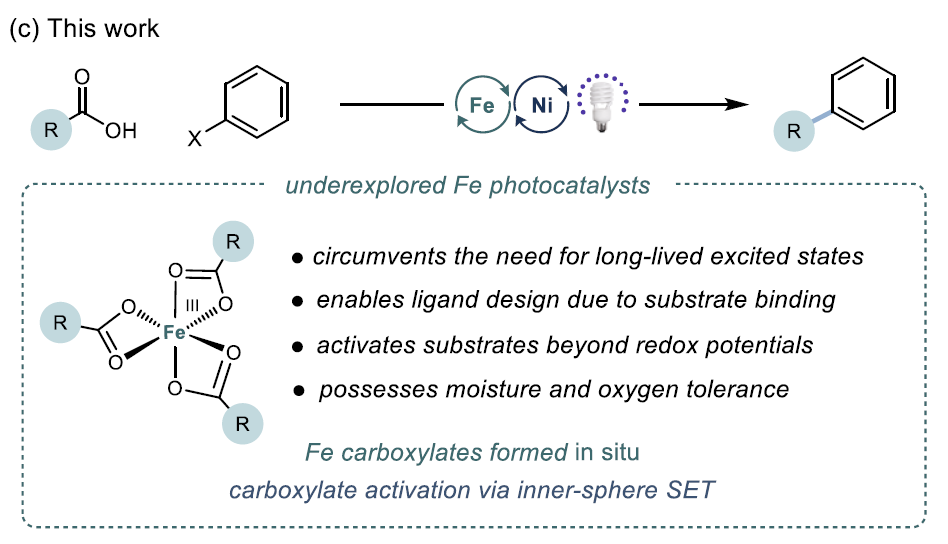 Decarboxylative cross-coupling is a key reaction for constructing C(sp²)-C(sp³) bonds, with traditional methods relying on precious metals (such as Ir, Ru) as photocatalysts, which are costly and require harsh conditions. Iron (Fe), as a cheap, abundant, and environmentally friendly metal, can achieve efficient photactivation of carboxylic acids through a ligand-to-metal charge transfer (LMCT) mechanism. This study reports the first successful decarboxylative coupling of aryl iodides with carboxylic acids by developing a Fe/Ni bimetallic photoredox system, breaking through the limitations of the traditional Ir/Ni system.
Decarboxylative cross-coupling is a key reaction for constructing C(sp²)-C(sp³) bonds, with traditional methods relying on precious metals (such as Ir, Ru) as photocatalysts, which are costly and require harsh conditions. Iron (Fe), as a cheap, abundant, and environmentally friendly metal, can achieve efficient photactivation of carboxylic acids through a ligand-to-metal charge transfer (LMCT) mechanism. This study reports the first successful decarboxylative coupling of aryl iodides with carboxylic acids by developing a Fe/Ni bimetallic photoredox system, breaking through the limitations of the traditional Ir/Ni system.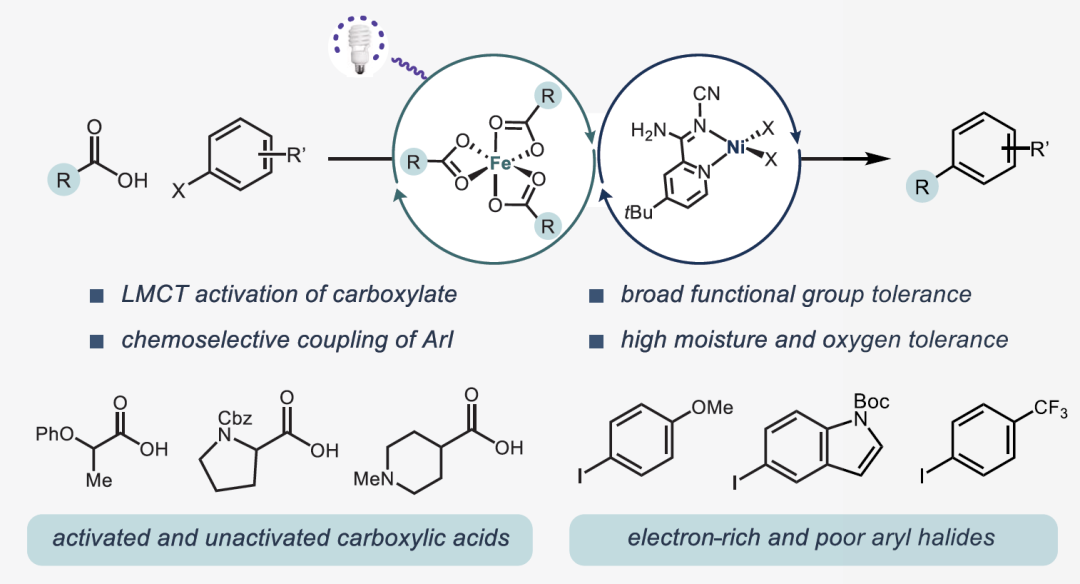 This article reports a decarboxylative cross-coupling method based on iron and nickel metal photoredox catalysis. The system utilizes inexpensive FeCl₃ as a co-catalyst and pyridyl carbamate nickel catalyst to achieve efficient coupling of aryl iodides with carboxylic acids, with excellent yields (23-96%), and demonstrates chemical selectivity towards boronic esters, trifluoromethanesulfonates, chlorides, and even bromides. The reaction is compatible with carboxylic acid derivatives containing heterocycles, N-protected amino acids, and protonic functional groups, suitable for sterically hindered, electron-rich, and electron-deficient aryl iodides. Preliminary catalytic and stoichiometric experiments support the following mechanism: under light irradiation, Fe is responsible for carboxylic acid activation, while the Ni¹ alkyl intermediate mediates the activation of aryl iodide and generates the product through reductive elimination.Catalyst DesignCore System: FeCl₃ (photocatalyst precursor) + pyridyl carbamate nickel complex (Ni/4-tBu PyCamCN).Ligand Screening: 4-tBu PyCamCN significantly enhances reaction efficiency (compared to the traditional 4-tBu Bpy ligand, yield increased from 37% to 86%).Additives: TBAI (tetrabutylammonium iodide) and phthalimide suppress side reactions (such as solvent C-H arylation).Reaction Conditions: 390 nm blue light irradiation, 1,4-dioxane solvent, 40°C, 24 hours
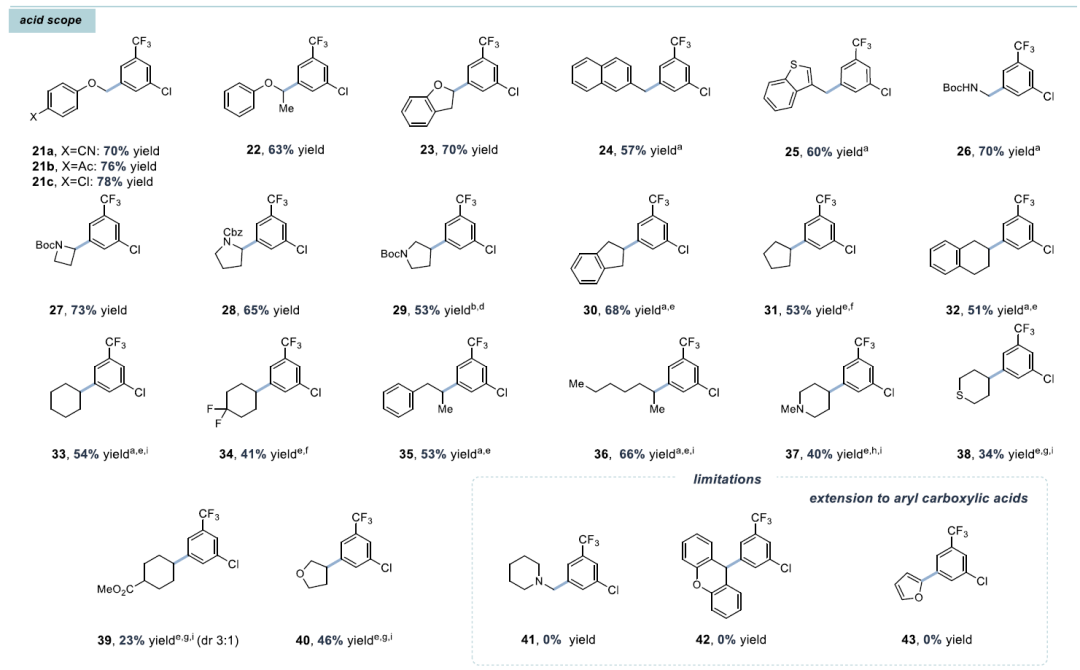
This article reports a decarboxylative cross-coupling method based on iron and nickel metal photoredox catalysis. The system utilizes inexpensive FeCl₃ as a co-catalyst and pyridyl carbamate nickel catalyst to achieve efficient coupling of aryl iodides with carboxylic acids, with excellent yields (23-96%), and demonstrates chemical selectivity towards boronic esters, trifluoromethanesulfonates, chlorides, and even bromides. The reaction is compatible with carboxylic acid derivatives containing heterocycles, N-protected amino acids, and protonic functional groups, suitable for sterically hindered, electron-rich, and electron-deficient aryl iodides. Preliminary catalytic and stoichiometric experiments support the following mechanism: under light irradiation, Fe is responsible for carboxylic acid activation, while the Ni¹ alkyl intermediate mediates the activation of aryl iodide and generates the product through reductive elimination.Catalyst DesignCore System: FeCl₃ (photocatalyst precursor) + pyridyl carbamate nickel complex (Ni/4-tBu PyCamCN).Ligand Screening: 4-tBu PyCamCN significantly enhances reaction efficiency (compared to the traditional 4-tBu Bpy ligand, yield increased from 37% to 86%).Additives: TBAI (tetrabutylammonium iodide) and phthalimide suppress side reactions (such as solvent C-H arylation).Reaction Conditions: 390 nm blue light irradiation, 1,4-dioxane solvent, 40°C, 24 hours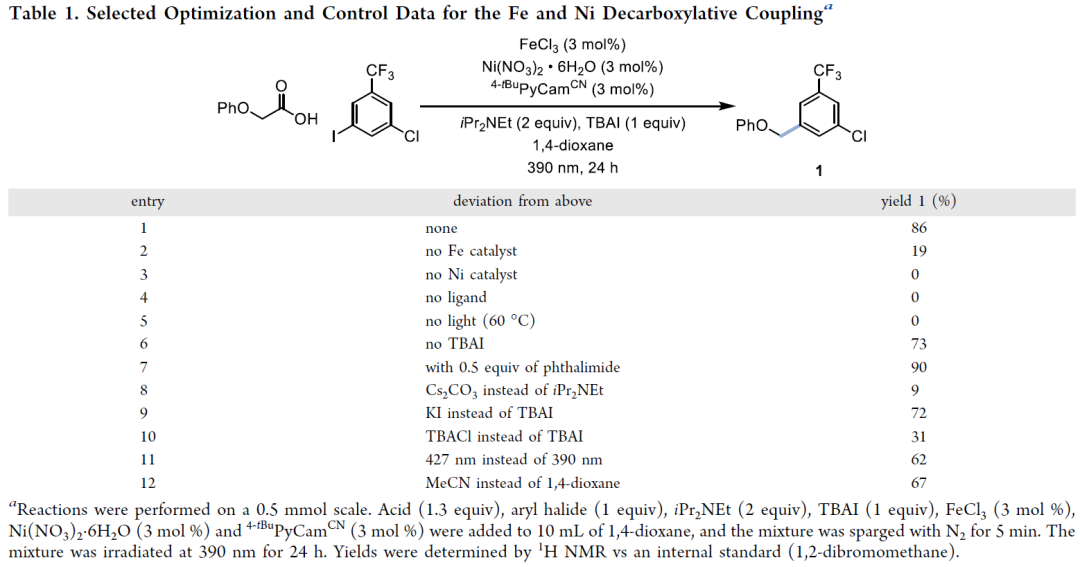 Substrate ApplicabilityThe first example of a Fe/Ni bimetallic photoredox system, replacing precious metal Ir, reduces costs (FeCl₃ costs only 0.008% of Ir catalyst). It exhibits high chemical selectivity, with C-I bonds preferentially reacting over C-Br/C-Cl/C-OTf, avoiding protection/deprotection steps. The substrate applicability is strong, accommodating unactivated carboxylic acids (such as cyclohexanoic acid) and sensitive functional groups (such as boronic esters, aldehydes). Strict deoxygenation/dehydration is not required, allowing operation under standard laboratory conditions.Aryl Iodides: 1. High selectivity, preferentially coupling C-I bonds (C-Br, C-Cl, C-OTf do not react); 2. Electronic diversity, tolerating substituents such as -CF₃ (92%), -OAc (60%), -F (65%), -OCF₃ (82%); 3. Heterocycle compatibility: furan (12), benzothiophene (13), indole (14), pyridine (15), etc.Carboxylic Acids: 1. Activated: α-heteroatom carboxylic acids (such as morpholinoacetic acid, yields 65-78%); 2. Unactivated: cyclopentanoic acid (41%), cyclohexanoic acid (68%), requiring addition of Zn or 4-ethylpyridine for optimization; 3. Amino acid derivatives: N-Boc glycine (66%), proline (53%).
Substrate ApplicabilityThe first example of a Fe/Ni bimetallic photoredox system, replacing precious metal Ir, reduces costs (FeCl₃ costs only 0.008% of Ir catalyst). It exhibits high chemical selectivity, with C-I bonds preferentially reacting over C-Br/C-Cl/C-OTf, avoiding protection/deprotection steps. The substrate applicability is strong, accommodating unactivated carboxylic acids (such as cyclohexanoic acid) and sensitive functional groups (such as boronic esters, aldehydes). Strict deoxygenation/dehydration is not required, allowing operation under standard laboratory conditions.Aryl Iodides: 1. High selectivity, preferentially coupling C-I bonds (C-Br, C-Cl, C-OTf do not react); 2. Electronic diversity, tolerating substituents such as -CF₃ (92%), -OAc (60%), -F (65%), -OCF₃ (82%); 3. Heterocycle compatibility: furan (12), benzothiophene (13), indole (14), pyridine (15), etc.Carboxylic Acids: 1. Activated: α-heteroatom carboxylic acids (such as morpholinoacetic acid, yields 65-78%); 2. Unactivated: cyclopentanoic acid (41%), cyclohexanoic acid (68%), requiring addition of Zn or 4-ethylpyridine for optimization; 3. Amino acid derivatives: N-Boc glycine (66%), proline (53%).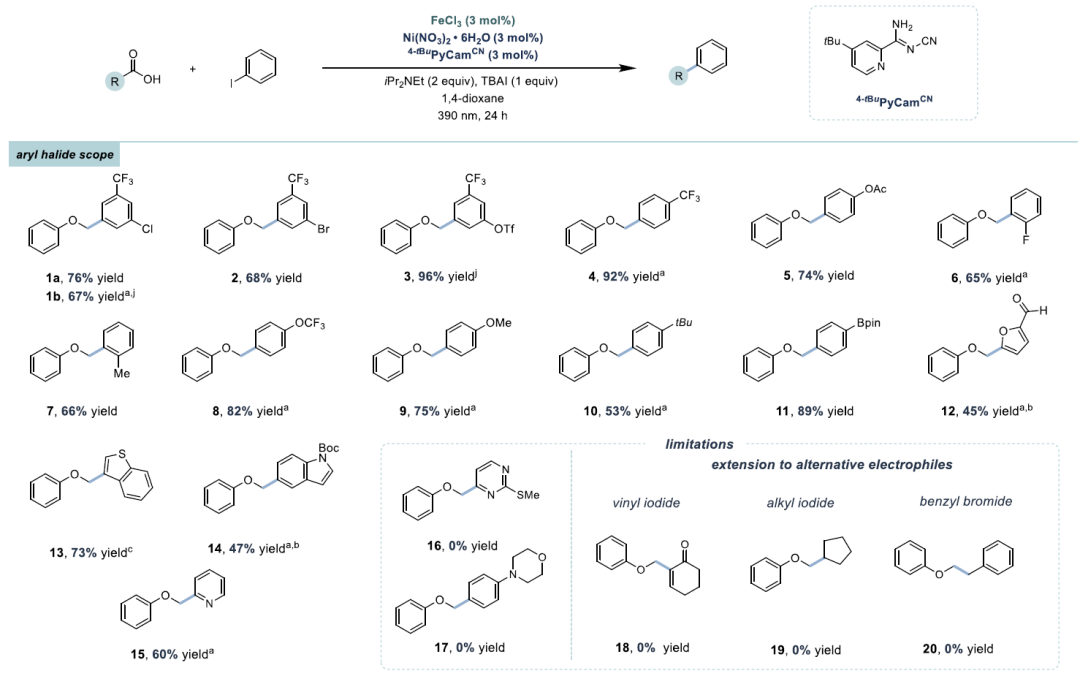
 Mechanistic StudyKey Evidence: Stoichiometric experiments confirm that the Ni(II) intermediate can oxidatively add to aryl iodide, supporting the Ni¹/Ni³ⁱ cycle pathway
Mechanistic StudyKey Evidence: Stoichiometric experiments confirm that the Ni(II) intermediate can oxidatively add to aryl iodide, supporting the Ni¹/Ni³ⁱ cycle pathway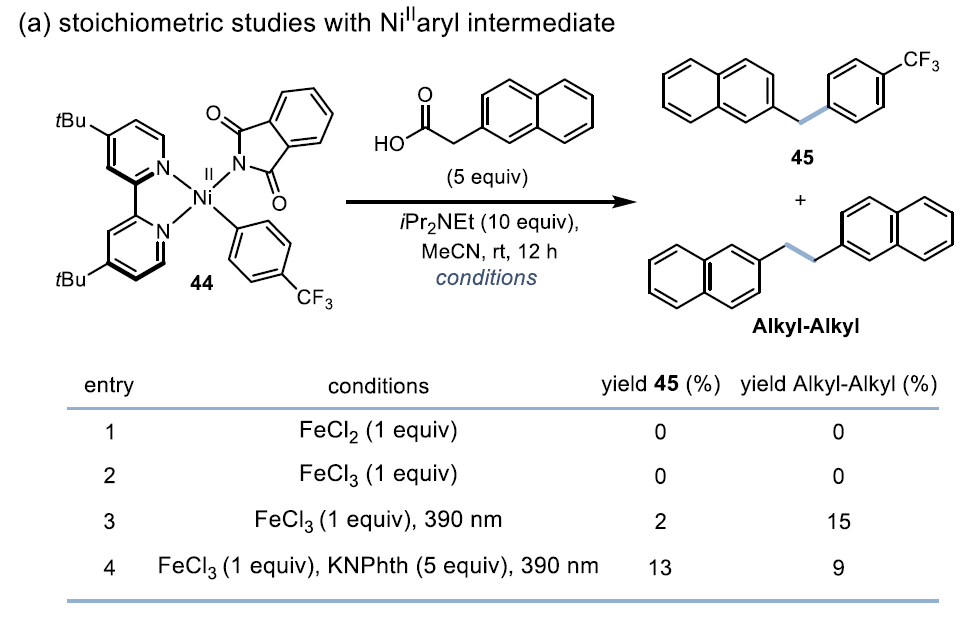
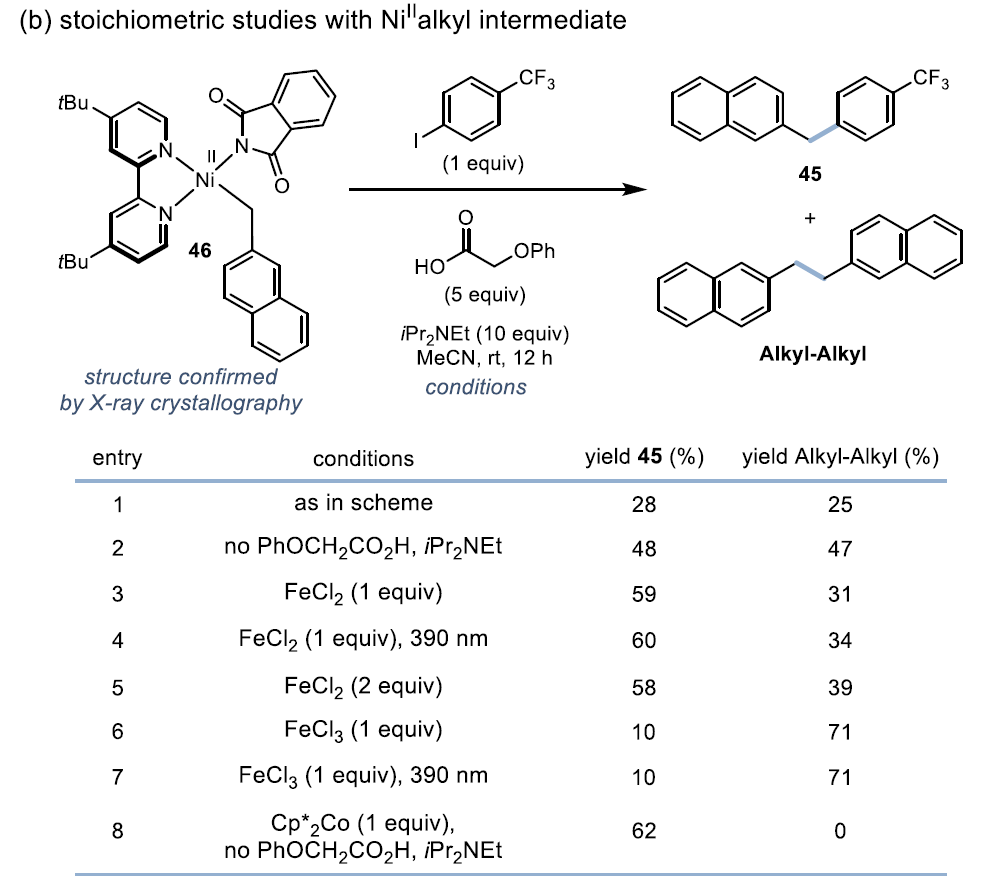 Fe/Ni-mediated decarboxylative arylation catalytic cycle. First, the Fe carboxylate complex (48) can be generated by the reaction of Fe salts with bases and carboxylic acids. Under 390 nm light irradiation, this complex undergoes a ligand-to-metal charge transfer (LMCT) event, producing carboxylate radical and Fe²⁺ species (49). Subsequently, the carboxylate radical rapidly decarboxylates and releases CO₂, generating an alkyl radical. This radical is captured by (Lₙ)Ni¹X (50), forming (Lₙ)Ni²⁺(alkyl)X intermediate (51). This species is reduced to (Lₙ)Ni¹(alkyl) (52) through single-electron reduction by either 49 or (Lₙ)Ni¹X. The reversible oxidative addition of aryl iodide forms (Lₙ)Ni³⁺(alkyl)(aryl)(I) (53), which then undergoes reductive elimination to generate the cross-coupling product and regenerate (Lₙ)Ni¹X (50).
Fe/Ni-mediated decarboxylative arylation catalytic cycle. First, the Fe carboxylate complex (48) can be generated by the reaction of Fe salts with bases and carboxylic acids. Under 390 nm light irradiation, this complex undergoes a ligand-to-metal charge transfer (LMCT) event, producing carboxylate radical and Fe²⁺ species (49). Subsequently, the carboxylate radical rapidly decarboxylates and releases CO₂, generating an alkyl radical. This radical is captured by (Lₙ)Ni¹X (50), forming (Lₙ)Ni²⁺(alkyl)X intermediate (51). This species is reduced to (Lₙ)Ni¹(alkyl) (52) through single-electron reduction by either 49 or (Lₙ)Ni¹X. The reversible oxidative addition of aryl iodide forms (Lₙ)Ni³⁺(alkyl)(aryl)(I) (53), which then undergoes reductive elimination to generate the cross-coupling product and regenerate (Lₙ)Ni¹X (50).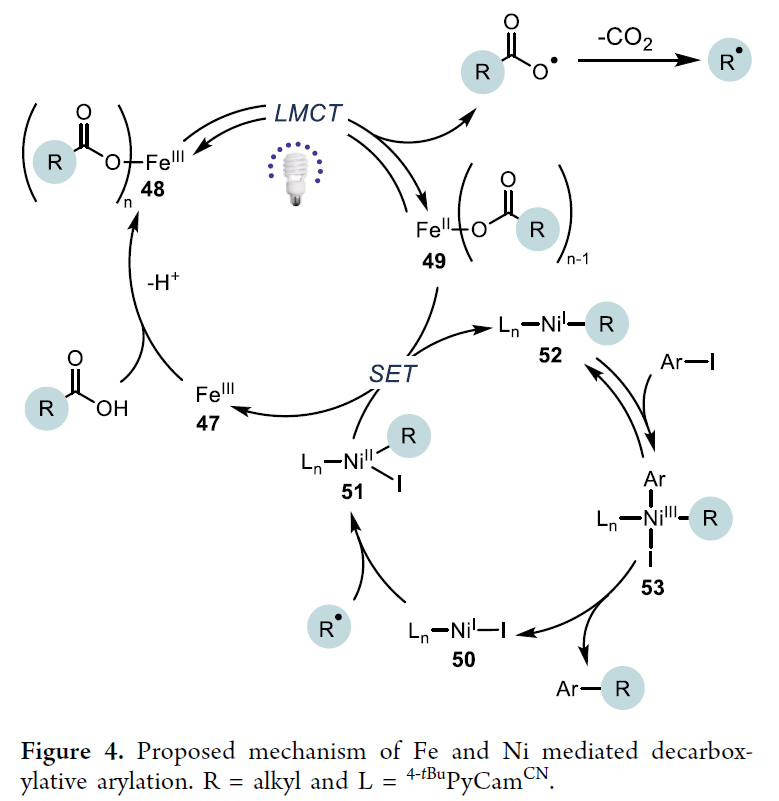 In summary, this article develops a decarboxylative cross-coupling method based on Fe/Ni metal photoredox. This system not only uses a low loading (3 mol%) of inexpensive metal catalyst but is also compatible with functional groups such as aldehydes, esters, and heterocycles, suitable for both activated and unactivated carboxylic acids. In the absence of aryl iodides, electron-deficient aryl bromides can also serve as coupling substrates. Mechanistic studies indicate that this reaction proceeds via the Ni¹/Ni³ⁱ oxidative addition pathway, which is distinctly different from the Ni⁰/Ni²ⁱ mechanism of the traditional Ir/Ni system. Future work will focus on further elucidating the Fe/Ni synergistic mechanism and expanding other cheap metal photoredox transformations.
In summary, this article develops a decarboxylative cross-coupling method based on Fe/Ni metal photoredox. This system not only uses a low loading (3 mol%) of inexpensive metal catalyst but is also compatible with functional groups such as aldehydes, esters, and heterocycles, suitable for both activated and unactivated carboxylic acids. In the absence of aryl iodides, electron-deficient aryl bromides can also serve as coupling substrates. Mechanistic studies indicate that this reaction proceeds via the Ni¹/Ni³ⁱ oxidative addition pathway, which is distinctly different from the Ni⁰/Ni²ⁱ mechanism of the traditional Ir/Ni system. Future work will focus on further elucidating the Fe/Ni synergistic mechanism and expanding other cheap metal photoredox transformations.