
【Copyright Statement】Original articles on this site are prohibited from being reproduced without permission. For reprints, please contact the copyright holder, De Daqi Equipment Industry Manager, and after obtaining permission, the source must be clearly indicated at the beginning of the article.

In recent years, surgical robots have been a hot topic, both within the industry and beyond. Of course, at the top of this field is the Da Vinci robot, known for its precision and high level of intelligence, which has been widely discussed. Indeed, Intuitive Surgical (the parent company of the Da Vinci robot) launched its first generation product at the end of the last century, and now it has been over20 years, accumulating a wealth of clinical and technical experience.


The first model: from the United States FLEXDEX Surgical Company FlexDex® wristed robotic arm (see product in Figure 1).
This device features a very unique user interface, where the device’s axis is connected to the user’s forearm via a bracelet connected to the tool frame, thus placing the wrist joint axis at the center of the surgeon’s wrist.
Figure 1 FlexDex instrument

The second model: from Germany Tuebingen Scientific Medical Company’s Radius Surgical System® (see product in Figure 2)
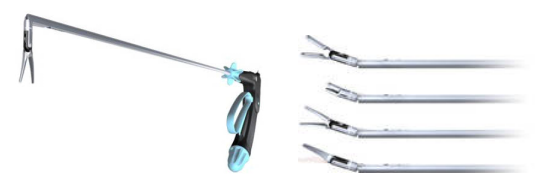
Figure 2 Radius Surgical System and its multifunctional attachments

The third model: from the Netherlands DEAM Company’s LaproFlex® instrument (see Figure 3)

Figure 3 LaproFlex® instrument

The fourth model: from the United States UNE Med Company’s Intuitool® instrument (see Figure 4)

Figure 4 Intuitool® instrument

The fifth model: from South Korea LIVSMED Company’s ArtiSential® instrument (see Figure 5)

Figure 5 ArtiSential® instrument

The sixth model: from France DEX Company’s Dex® instrument (see Figure 6)

Figure 6 Dex® instrument

The seventh model: from China’s Suzhou Kando Robot Company’s micro laparoscopic surgical robot (see Figure 7)

Figure 7 Kando® micro laparoscopic surgical robot
Previous Content Review
Summary of Guidelines and Regulations
【Weekly Learning a Guideline】 Registration Technical Review Guidelines for Oral and Maxillofacial Cone Beam Computed Tomography Equipment
【Weekly Learning a Guideline】 Registration Technical Review Guidelines for Light Curing Machines
【Weekly Learning a Guideline】 Registration Technical Review Guidelines for Electric Hospital Beds
【Weekly Learning a Guideline】 Clinical Trial Guidelines for Cochlear Implant Systems
【Weekly Learning a Guideline】 Registration Technical Review Guidelines for Rigid Optical Endoscopes (Class II)
【Latest Regulations Summary】 National Day Seven Days of Fun Regulations Update (National Bureau Big Gift Pack)
Latest Heavyweight! IVDR Officially Announced Postponement (with Regulatory Amendments)
Medical Device Literature Society
【Medical Device Literature Society】 In such an “involution” system, it is too difficult for process technicians.
【Medical Device Literature Society】 Medical Aesthetic Chaos Needs to be Rectified, Beauty Rights Must Be Guaranteed
【Medical Device Literature Society】 A Brief Discussion on AI Applications in the Field of Medical Devices
【Medical Device Literature Society】 Key Elements for Ensuring Quality System Operation: Self-Inspection (II)
【Medical Device Literature Society】 Sources and Control Methods for Microbial Contamination in Production Environments
【Medical Device Literature Society】 Key Elements for Ensuring Quality System Operation: Self-Inspection (I)
【Medical Device Literature Society】 Don’t Let Communication Affect Work Quality
【Medical Device Literature Society】 Quality Control – A Brief Discussion on the Validation of Initial Bacterial Inspection Methods
【Medical Device Literature Society】 How to Avoid Expired Materials Being Used from the Warehouse
【Medical Device Literature Society】 A Brief Discussion on the Causes and Prevention of Biofilm Formation in Water Systems
Knowledge Sharing
【Knowledge Sharing】 How to Conduct Internal Audits in Enterprises
【Knowledge Sharing】 Basic Principles of Medical Device Safety and Performance (Safety and Efficacy Basic Requirements) Checklist Revision Comparison【Knowledge Sharing】 Why Should Clean Areas Have Buffer Devices?
【Knowledge Sharing】 CRF (Case Report Form) Filling Guidelines
【Knowledge Sharing】 Key Points for Writing the “Periodic Risk Assessment Report” for Medical Devices
【Knowledge Sharing】 How to Manage Medical Device Suppliers
【Knowledge Sharing】 Key Points for Sample Retention of Medical Device Products
【Knowledge Sharing】 Summary: Analysis Parameter Settings for Fully Automated Biochemical Analyzers
【Knowledge Sharing】 How CRC Assists in Handling Overheating of Investigational Drugs
【Knowledge Sharing】 How to Rectify Non-Conformities in Quality System Internal Audits?
Clinical Relevance
The New Version of “Technical Guidelines for Clinical Trials of In Vitro Diagnostic Reagents” is Officially Released and Implemented Discussion on Risk Management in Clinical Trials of Aesthetic and Cosmetic Medical Devices
The National Standardization Technical Unit for Clinical Evaluation of Medical Devices is Established
【Key Points】 How to Ensure Compliance in Clinical Trials of Medical Devices
What are the Requirements for Submitting Clinical Trial Data for Medical Devices? What are the Similarities and Differences Compared to Drug Clinical Trials?
【Supervision and Inspection】 Announcement on the Supervision and Inspection of Clinical Trials and Institutions by the Shanghai Drug Administration in 2021
【Medical Device Literature Society】 These are the Things We Should Know About Clinical Trial Grouping!
【In Vitro Diagnostics】 Research on Quality Assurance Measures for Clinical Trials of In Vitro Diagnostic Reagents
【Original】 A Brief Discussion on Key Points for On-site Inspection of Medical Devices: Informed Consent, Informed Consent Form (2.1)
【Original】 A Brief Discussion on Key Points for On-site Inspection of Medical Devices: Informed Consent, Informed Consent Form (2.2) Template Included!
Training Series
【Post-Class Highlights Sharing】 “How to Conduct Clinical Evaluation Under the New Regulations”
【Post-Class Highlights Sharing】 “Implementation and Management Application of Unique Device Identification (UDI)”
【Post-Class Highlights Sharing】 “Compliance Planning for Medical Device Quality Management Systems”
【Post-Class Highlights Sharing】 “Introduction to Sterile Medical Device Packaging”
【Post-Class Highlights Sharing】 “Discussing Medical Device R&D from a Project Management Perspective”
【Post-Class Highlights Sharing】 “Validation Tests for Sterile Medical Device Transport”
【Post-Class Highlights Sharing】 “Ethylene Oxide Sterilization”
【Post-Class Highlights Sharing】 “Application of Risk Management in Medical Devices”
【Post-Class Highlights Sharing】 “Basic Requirements for Medical Device Software Registration”

END





