Open the public account and set it as a “star”.
Follow us to learn more about cancer information!~
Breast Cancer
In recent years, the treatment of breast cancer has been influenced by the practice of precision oncology, requiring personalized medical care based on specific tumor characteristics. The treatment of breast cancer primarily depends on the expression results of estrogen receptor (ER), progesterone receptor (PR), and Her2/neu (HER2) protein. However, due to tumor heterogeneity, these prognostic markers may vary significantly even within a single biopsy. Additionally, many retrospective studies have shown that prognostic markers may undergo transformation during tumor progression, making it crucial to accurately identify prognostic markers to provide appropriate targeted therapy.
The case shared today introduces a breast cancer patient with HER2 negative status in the primary tumor, who rapidly progressed under various treatment methods, and was later re-tested for metastases through NGS, confirming HER2 positive status. After receiving anti-HER2 treatment, the patient exhibited an excellent clinical response, improving her quality of life.
Case Introduction

In October 2017, a 54-year-old female patient presented with a one-year history of back pain, new left breast tenderness, and nipple retraction. The left breast ultrasound-guided biopsy revealed invasive lobular carcinoma, grade 2, moderately differentiated, ER strong positive 90%, PR strong positive 95%, HER2 negative (1+), and low Ki-67 10%. PET/CT scan showed left iliac crest bone lesions, and biopsy confirmed metastatic disease, ultimately diagnosing her with newly diagnosed stage IV breast cancer (cT2, cN1, cM1, G2). At diagnosis, the patient’s initial Karnofsky performance status (KPS) was 90.
Additionally, due to the patient’s family history, she underwent BRCA1/2 gene testing: an aunt with breast cancer, a cousin diagnosed with breast cancer (BRCA1 mutation, del exons 13-24 heterozygous), and a cousin diagnosed with ovarian cancer. The results showed that the patient did not carry pathogenic mutations, with BRCA1 and BRCA2 being negative, but mutations in PIK3CA, AKT3, CDH1, MAP3K1, and NOTCH4 were detected.
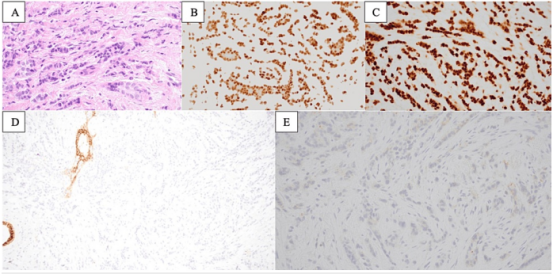
Figure 1: Histological sections show invasive lobular carcinoma (A, 400x). Immunohistochemistry for estrogen receptor (B, 400x) and progesterone receptor (C, 400x) both show strong positivity in cancer cells. E-cadherin negative (D, 200x), supporting lobular phenotype; HER2 negative (1+) (E, 400x).
The patient’s initial first and second-line treatments did not include targeted therapy. After disease progression, the patient began third-line treatment and participated in a phase II clinical trial (NCT04090398), being randomly assigned to the paclitaxel monotherapy group. Two months later, imaging continued to show disease progression, and the patient stopped participating in the clinical trial.
Subsequently, the patient’s blood was tested through liquid biopsy, detecting PIK3CA E542K and CDH1 L725fs mutations, as well as synonymous changes in TP53 E224D and MAPK1 I56I. The patient then received fourth-line treatment with 250 mg of Alpelisib daily and 20 mg of Tamoxifen daily for six months.
However, follow-up PET/CT revealed disease progression, and the patient underwent cervical and brain MRI scans, which showed diffuse leptomeningeal disease. Cerebrospinal fluid samples underwent NGS testing, revealing HER2 amplification. Meanwhile, liver biopsy pathology confirmed metastatic breast cancer (with pleomorphic lobular features), E-cadherin negative, GATA3 positive, TRPS1 positive, ER weakly positive 11-50%, PR weakly positive 11-50%, and HER2 positive (3+).
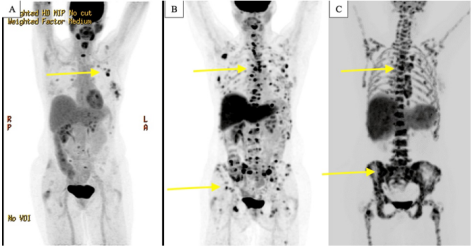
Figure 2:PET/CT scans performed at different time points during disease progression. (A) December 2020, increased fluorodeoxyglucose (FDG) activity in the left breast, axillary lymph nodes, and spine. (B) June 2021, worsening metabolic activity in the thoracolumbar and pelvic bones. (C) February 2022, further progression due to multiple mediastinal and abdominal lymphadenopathy and extensive skeletal metastases in the axial and appendicular skeleton.
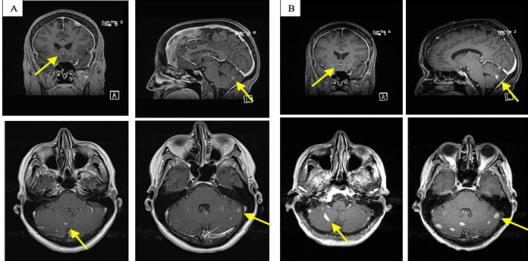
Figure 3: (A) Brain MRI obtained in January 2022 shows thickening of the meninges, with focal meningeal lesions in the left frontal lobe and multiple enhancing lesions in the posterior fossa. (B) Follow-up MRI obtained in March 2022 shows an increase in size and number of brain lesions in the posterior fossa, with worsening meningeal enhancement.
Due to the newly discovered HER2 positivity, the patient received capecitabine + tucatinib + trastuzumab treatment, but due to adverse reactions from side effects, the patient attempted DS-8201 for sixth-line treatment. During treatment, the patient tolerated the therapy well, and her physical condition improved from KPS 50 to KPS 90. Follow-up brain MRIs at 1 month, 3 months, 7 months, and 10 months after starting DS-8201 treatment showed stable or slightly improved cerebellar metastases, with no new metastatic lesions and persistent stable mild diffuse meningeal enhancement.
Thirteen months later, MRI showed progression of leptomeningeal disease, and the patient passed away in August 2023.
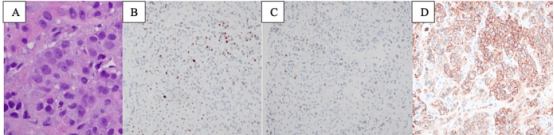
Figure 4: Pathological sections from liver biopsy in February 2022 show pleomorphic carcinoma involvement (A; 600x); tumor cells show weak positivity for estrogen receptor (subgroup, weak) (B, 400x); immunohistochemical progesterone receptor weak positivity (C, 400x); HER2 immunohistochemistry positive (3+) (D, 400x)
A retrospective study of 104 patients in Sweden showed that nearly 15% of patients experienced a change in HER2 status from the primary tumor to recurrence. Another retrospective study of 110 patients from Greece showed that 18% of patients had inconsistent HER2 expression in the primary tumor, accompanied by recurrence or metastasis. Therefore, it is important to repeat biopsies for patients with poor treatment response to determine whether tumor characteristics have evolved.
DS-8201 has been shown to have significant antitumor activity in treated patients with metastatic HER2-positive breast cancer. Additionally, DS-8201 has also been approved for HER2-low (1+) metastatic breast cancer, showing a longer progression-free survival (9.9 months vs 5.1 months) and overall survival (23.4 months vs 16.8 months) compared to chemotherapy selected by physicians. DS-8201 is also capable of penetrating the blood-brain barrier, demonstrating special efficacy in the treatment of brain metastases from breast cancer.
In summary, this case highlights the importance of subsequent tumor testing and the advantages of using NGS to identify new targets and potential treatment options. Therefore, it is crucial to continuously track changes in the patient’s tumor and related molecular markers throughout the disease course to seek more treatment opportunities.
Case Reading Tips:

✦
•
1. Cancer has the potential for continuous evolution, and re-biopsy can continuously track the patient’s pathology, obtaining different test results, which is beneficial to ensure the correct targeted therapy is provided to the patient.
✦
•
2. DS-8201 still has significant antitumor activity in treated patients with metastatic HER2-positive breast cancer.
✦
•
3. Breast cancer patients with relevant personal or family history suggest genetic susceptibility to breast cancer, and screening for germline variants including BRCA1/2, PALB2 and other genetic susceptibility genes is recommended.
✦
•
4. For patients with brain metastases, cerebrospinal fluid NGS testing is a sample type that should not be overlooked.
References: Seraphina, Choi., Daniel, Cassidy., Patricia, Castillo., Eric A, Mellon., Carmen, Calfa.(2024). Cerebrospinal Fluid Testing in Leptomeningeal Progression of HER2-Negative Breast Cancer Reveals HER2 Positivity, Leading to HER2-Targeted Therapy: A Case Report. Cureus, 16(3), 0. doi:10.7759/cureus.55483

Warm Reminder
Due to WeChat’s modification of push rules, if you do not click “Looking”, you will gradually stop receiving our pushes.
Please add “Yunying” as a star, and don’t forget to click “like” and “Looking” after reading each time.
About
Yunying Medical
YUNYING
Yunying Medical is centered in the Yangtze River Delta region, radiating across China. The company is committed to creating a new ecosystem for precision cancer diagnosis and treatment, comprehensively utilizing cutting-edge technologies such as gene mutation detection, gene methylation detection, and gene editing recombination, providing integrated tumor precision medicine solutions from early cancer screening, targeted drug testing, to cancer treatment drug development. Currently, there are three laboratories located in Shanghai, Zhejiang, and Qinghai.
Since its establishment, Yunying Medical has successfully passed nearly a hundred inter-laboratory quality assessments organized by institutions such as the National Health Commission’s Clinical Inspection Center, the National Pathology Quality Control Center, and the American Society of Pathology. The testing capabilities have been accredited by the College of American Pathologists (CAP), and the tumor core gene testing kit has received EU CE certification, obtained registration approval from the National Medical Products Administration (NMPA), and the production management quality system has been certified by international ISO13485. Currently, Yunying has provided precision testing services for nearly 300,000 cancer patients, pioneering the field of precision medicine for cancer in China. At the same time, Yunying adheres to the philosophy of “rigorous and efficient,” committed to the innovation and application exploration of molecular diagnostic technology, contributing to the robust development of precision medicine and companion diagnostics in oncology.