In an article published in the journal Science, a research team from the United States developed a platform called Imaging-Guided In Vivo Acoustic Printing (DISP). This technology aims to overcome the limitations of traditional 3D printing in medical applications, particularly the need for invasive surgery. DISP achieves precise and rapid crosslinking of biomaterials in deep tissues of living animals by using low-temperature sensitive liposomes as carriers, combined with polymer bio-inks and focused ultrasound technology. This method not only allows real-time monitoring of the printing process but also successfully implements in vivo printing in mouse bladders and rabbit leg muscles, demonstrating its potential in localized drug delivery and tissue replacement.
In an article published in the journal Science, a research team from the United States developed a platform called Imaging-Guided In Vivo Acoustic Printing (DISP). This technology aims to overcome the limitations of traditional 3D printing in medical applications, particularly the need for invasive surgery. DISP achieves precise and rapid crosslinking of biomaterials in deep tissues of living animals by using low-temperature sensitive liposomes as carriers, combined with polymer bio-inks and focused ultrasound technology. This method not only allows real-time monitoring of the printing process but also successfully implements in vivo printing in mouse bladders and rabbit leg muscles, demonstrating its potential in localized drug delivery and tissue replacement.
01

Research Background
Three-dimensional (3D) bioprinting technology has shown tremendous transformative potential in the medical field, enabling the customization of implants, complex medical devices, and tissue substitutes for patients. However, these constructed implants often require invasive surgery, limiting their application in minimally invasive treatments. Although near-infrared (NIR) light has been used as a biocompatible energy source for in vivo printing, its application is limited to subcutaneous tissues due to the limited penetration depth of light. Ultrasound technology, with its ability to penetrate deep tissues and its non-invasive characteristics, provides a promising platform for in vivo printing. Its real-time imaging capability allows for precise localization and control during the in situ manufacturing of biomaterials.In in vivo printing technology, the main challenge lies in developing multifunctional bio-ink formulations that can adapt to various biomaterials to ensure broad applicability in different medical scenarios while ensuring high biocompatibility and minimal residual prepolymer toxicity. To address these issues, comprehensive in vitro and in vivo studies are essential. Furthermore, advancing these technologies requires systems capable of large-scale and high-resolution printing, seamlessly integrated with real-time imaging to ensure precise focal localization, minimize the impact on non-target tissues, and accelerate clinical translation. The research team developed an Imaging-Guided In Vivo Acoustic Printing (DISP) platform that utilizes low-temperature sensitive liposomes (LTSLs) as carriers to deliver crosslinking agents for precise and controllable in situ manufacturing of biomaterials in deep tissues.
02

Research Findings
This paper developed a platform called DISP (Deep In Vivo Acoustic Printing), which utilizes imaging-guided ultrasound printing technology to achieve precise manufacturing of biological structures in deep tissues in vivo. The research team integrated low-temperature sensitive liposomes (LTSLs) as carriers for crosslinking agents into the bio-ink, utilizing focused ultrasound (FUS) to achieve rapid, on-demand crosslinking of various functional biomaterials. A significant advantage of the DISP platform is its ability to perform real-time monitoring and customized pattern creation in living animals, successfully conducting in vivo printing in mouse bladders and rabbit leg muscles, demonstrating its potential in localized drug delivery and tissue replacement.The DISP platform can achieve high resolution (approximately 150 micrometers) and fast printing speeds (up to 40 millimeters per second), successfully printing various functional biomaterials, including conductive, drug-loaded, cell-loaded, and bioadhesive hydrogels. By integrating gas vesicle (GV)-based ultrasound imaging, the DISP platform can perform real-time monitoring during the printing process, ensuring precise focal localization and verification of in vivo crosslinking. The research also indicates that the application of DISP technology in living animals exhibits high biocompatibility, and the printed hydrogel structures demonstrate good stability and functionality within tissues. The successful application of this technology showcases its broad potential in various biomedical applications, including bioelectronics, drug delivery, tissue regeneration, and wound sealing.
03

Clinical Significance
Minimally invasive implantation and treatment: The DISP technology allows for direct printing of biological structures at specific sites in vivo without the need for surgery. This is particularly important in cases requiring rapid repair and replacement of tissues, such as tissue regeneration, drug delivery, and wound sealing. Multifunctional biomaterial applications: The research demonstrates that DISP technology can print conductive, drug-loaded, cell-loaded, and bioadhesive hydrogel materials. This versatility offers vast possibilities for personalized medical applications, including the preparation of bioelectronic devices, localized drug delivery systems, and tissue regeneration platforms. Real-time imaging and precise control: DISP integrates gas vesicle-based ultrasound imaging technology, allowing real-time monitoring of the printing process, ensuring precision and high resolution in printing. This provides assurance of accuracy in clinical applications, reducing potential damage to healthy tissues. Biocompatibility and safety: In vivo experiments of the study indicate that the hydrogels printed using this technology exhibit good biocompatibility and no significant toxic reactions. This strongly supports the safety of its clinical applications. Potential application areas: The research successfully demonstrates the ability to perform in vivo printing in mouse bladders and rabbit leg muscles, showcasing the immense potential of this technology in therapeutic interventions and tissue replacement. Overall, the DISP technology opens new avenues for bioprinting in minimally invasive medical treatments, with significant clinical application potential, especially in medical scenarios requiring precise, rapid, and safe tissue repair and replacement.
04

Experimental Strategy
1. Design and use of bio-inks: Using low-temperature sensitive liposomes (LTSLs) as carriers for crosslinking agents, which are activated at temperatures slightly above body temperature to release the crosslinking agents. The bio-ink (US-ink) consists of biopolymers, crosslinker-encapsulated LTSLs, and gas vesicles (GVs), which serve as contrast agents for ultrasound imaging.2. Application of focused ultrasound technology: Using focused ultrasound (FUS) technology, the FUS focal point is precisely located on the US-ink through an automatic positioning system. FUS induces localized heating, causing LTSLs to release crosslinking agents, achieving instant in situ crosslinking of US-ink.3. Imaging and monitoring: The integrated gas vesicle (GV)-based ultrasound imaging technology provides real-time monitoring, ensuring an accurate printing process. Real-time imaging via ultrasound allows for precise monitoring of the printing process and crosslinking verification.4. Experimental validation and application: In vivo experiments were conducted in mouse bladders and rabbit leg muscles to validate the practicality of DISP. Successful printing of conductive, drug-loaded, cell-loaded, and bioadhesive hydrogels demonstrated the potential of DISP in various biomedical fields such as bioelectronics, drug delivery, and tissue regeneration.
05

Data Interpretation
Figure 1: Imaging-Guided In Vivo Deep Tissue Acoustic Printing (DISP)
Figure 1 illustrates how the DISP platform constructs precise functional biological structures non-invasively in vivo using ultrasound ink (US-ink). The system combines gas-based ultrasound imaging to monitor target organs, detect the presence of prepolymers, and ensure accurate localization and successful ultrasound gel formation. A. Schematic diagram of the DISP platform. The DISP system uses ultrasound ink composed of un-crosslinked prepolymers, temperature-sensitive liposomes (LTSLs) carrying crosslinking agents, and gas vesicles. The ultrasound ink is injected into the body for non-invasive construction of precise functional biological structures. Integrated gas-based ultrasound imaging is used to monitor target organs, detect the presence of prepolymers, and ensure accurate localization and successful ultrasound gel formation. B. In vivo printing setup for generating and monitoring focused ultrasound (FUS). RF represents radio frequency, T/R represents transmitter/receiver. C. Transmission electron microscopy (TEM) image of temperature-sensitive liposomes (LTSLs) carrying crosslinking agents. Scale bar: 100 nanometers. D. Scanning electron microscopy (SEM) image of freeze-dried 3D printed alginate ultrasound gel. Scale bar: 20 micrometers. E. Functional hydrogel structures printed in vivo using ultrasound. Scale bar: 5 millimeters. F-H. In vivo printing based on DISP for biosensors and recording (F), biocarriers for drug delivery and tissue regeneration (G), and bioadhesives for wound sealing and device/tissue interfaces (H). Conclusion: The DISP platform can non-invasively construct various functional biological structures in vivo, including bioelectronic devices, biocarriers, and bioadhesives, demonstrating its potential in medical applications.
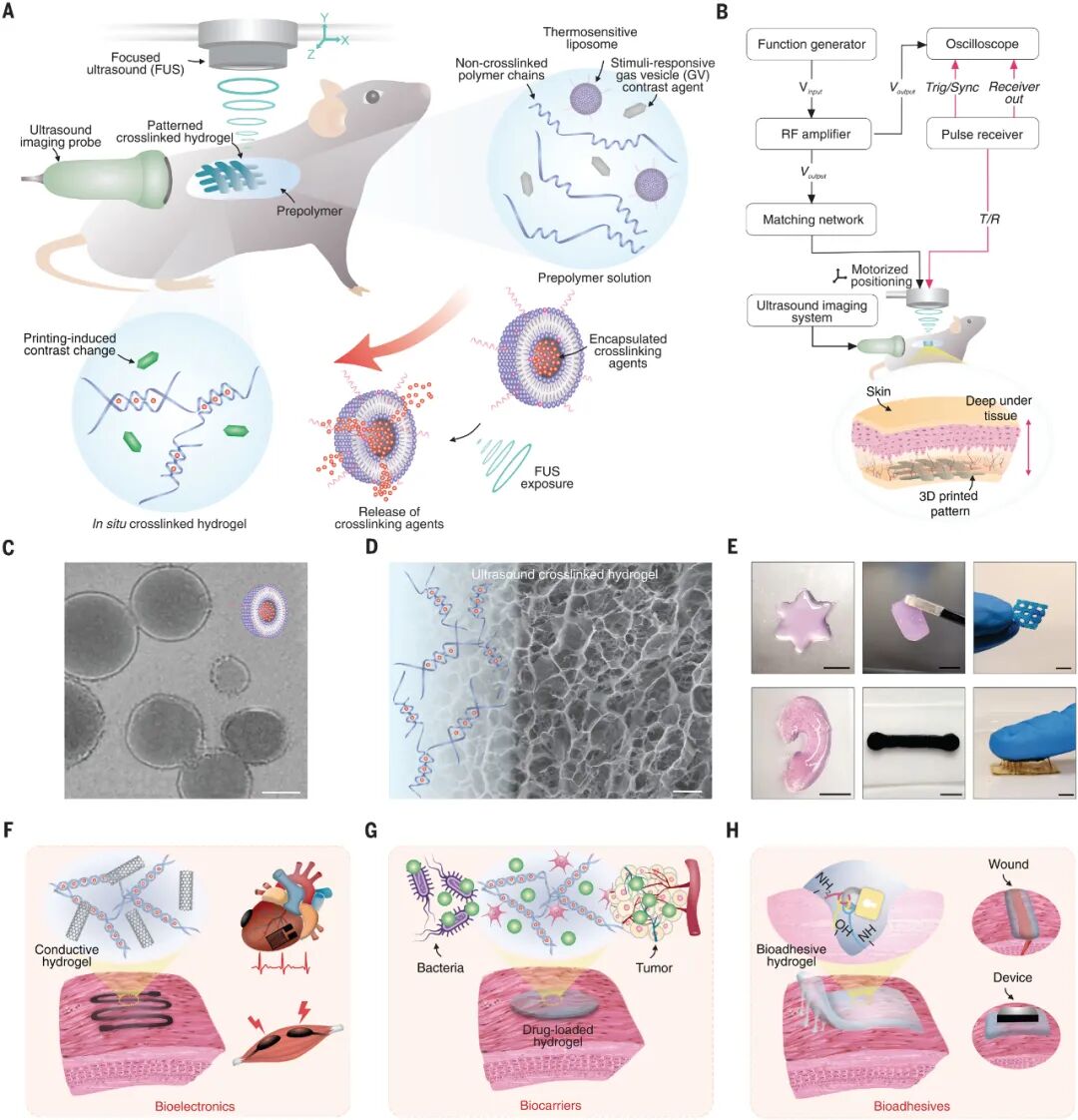
Figure 2: Synthesis and characterization of low-temperature sensitive liposomes for controlled release of crosslinking agents
Figure 2 illustrates the synthesis of low-temperature sensitive liposomes (LTSLs) and their application in the release of crosslinking agents. A. A schematic diagram shows the phase transition process of LTSLs from solid to liquid at slightly elevated temperatures, leading to the formation of nanopores in the lipid bilayer. B. Large-scale production of LTSLs loaded with crosslinking agents (e.g., Ca2+) was achieved through extrusion processes. C. Dynamic light scattering (DLS) analysis showed changes in the size of LTSLs before and after extrusion, indicating size variation post-extrusion. D. Fluorescence imaging using fura-2-acetoxymethyl ester as an intracellular calcium indicator demonstrated the presence of Ca2+ in LTSLs. E. Stability studies showed the release of Ca2+ from LTSLs stored at 4°C and 25°C after 1 month and 6 months, indicating the effect of temperature on Ca2+ release. F. UV-visible analysis studied the changes in LTSLs at 43°C over different time intervals, showing the temperature effect on LTSLs. G. Temperature-dependent Ca2+ release from LTSLs at 43°C and 37°C was studied, showing more significant release at 43°C. H. The crosslinking time of alginate US-ink was studied at different heating temperatures with a fixed LTSL concentration of 50 wt%, showing the effect of temperature on crosslinking time. I. The ionic crosslinking of alginate US-ink at different LTSL concentrations was evaluated through storage modulus (Gʹ) and loss modulus (Gʹʹ), with illustrations showing the gel state of alginate US-ink containing 0%, 15%, and 50% LTSLs after 30 seconds of exposure at 43°C. J. The crosslinking time of alginate US-ink at different LTSL concentrations was measured, showing the effect of LTSL concentration on crosslinking time. K. Live/dead staining imaging observed the state of human dermal fibroblasts cultured for 7 days in alginate, alginate US-ink containing 50% LTSLs, and alginate US-gel, showing cell viability. Conclusion: Low-temperature sensitive liposomes can effectively control the release of crosslinking agents during temperature changes and exhibit good performance in the crosslinking process of biomaterials.
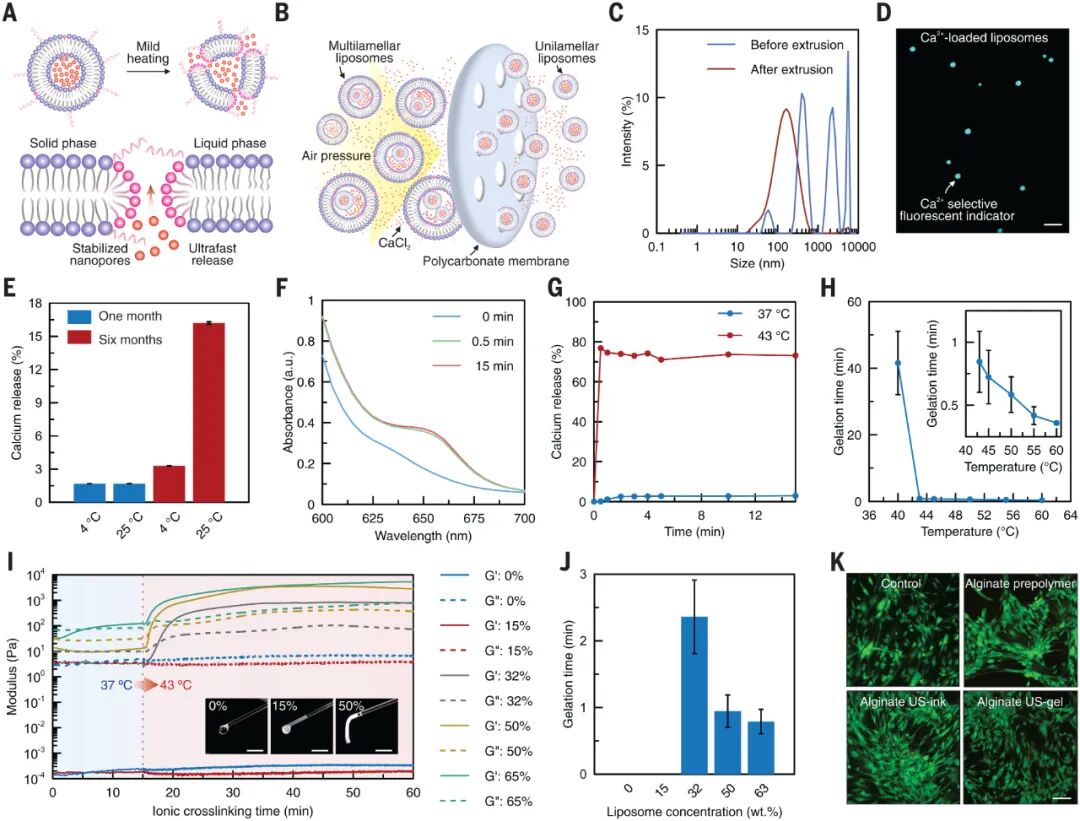 Figure 3: Characteristics of Focused Ultrasound-Induced 3D Printing
Figure 3: Characteristics of Focused Ultrasound-Induced 3D Printing
Figure 3 illustrates the application characteristics of focused ultrasound (FUS) in 3D printing, including its penetration depth, temperature distribution, pressure distribution, and printing resolution. A. A schematic diagram shows the process of focused ultrasound wave propagation, illustrating its precise localization capability on ultrasound ink (US-ink). B. Comparison of ultrasound with various light sources (e.g., UVA, UVB, and near-infrared light) in terms of penetration depth in tissues, showing superior penetration capability of ultrasound and indicating an inverse relationship between ultrasound frequency and penetration depth. C. Thermal simulations demonstrated the temperature distribution at the focal point under different frequencies and exposure times, with a scale bar of 2 millimeters. D. The temperature change curve at the focal point during and after 10 seconds of exposure to focused ultrasound at 8.75 MHz frequency. E-H. Experimental measurements and simulation results conducted in the X-Z and X-Y planes using a 2.65 MHz transducer, showing standardized pressure distribution maps at the focal point. E and F are experimental measurement results, while G and H are simulation results. I. Patterns of ultrasound gel (US-gel) printed using DISP technology, with an illustration scale bar of 400 micrometers, and the right pattern scale bar of 4 millimeters. J. Evaluating the printability of alginate ultrasound ink at different power levels and printing speeds using an 8.75 MHz transducer. K. Measuring the printing resolution of alginate ultrasound ink at different power levels and printing speeds using an 8.75 MHz transducer, represented in line width, with a scale bar of 5 millimeters. L. Measuring the printing resolution of alginate ultrasound ink under 15 mm thick pork loin tissue at 18 W power, different frequencies, and printing speeds, represented in line width. The illustration shows deep printed patterns on pork tissue, with a scale bar of 5 millimeters. M. Using DISP technology, the dissociation process of patterned alginate ultrasound gel on tissue was demonstrated after 5 minutes of treatment with a 0.025 M EDTA solution. The error bars in the figure represent the standard deviation of the mean (n = 3). Conclusion: Focused ultrasound exhibits precise localization capability, superior tissue penetration, controllable temperature and pressure distribution, as well as good printing resolution and controllable gel dissociation characteristics in 3D printing.
 Figure 4: Applications of Functional Biomaterials 3D Printing Technology Based on Deep Tissue In Vivo Acoustic Printing in Various Medical Applications
Figure 4: Applications of Functional Biomaterials 3D Printing Technology Based on Deep Tissue In Vivo Acoustic Printing in Various Medical Applications
Figure 4 A. To illustrate the composition of conductive US-ink, the authors provide a schematic diagram showing the entanglement of carbon nanotube (CNT) additives in alginate US-ink, crosslinked via focused ultrasound (FUS). B. Stability testing of the printed conductive US-gel pattern under cyclic bending deformation showed stable electrical performance. C. Temperature sensing tests using the printed conductive US-gel showed consistent and reversible responses when in contact with human skin. D. Conductive US-gel sensors printed using DISP technology were used for electrocardiogram (ECG) and electromyogram (EMG) recordings in human participants. E. Therapeutic biomolecules were integrated into US-ink to form biocarrier US-gel, demonstrating its potential for drug delivery applications. F. The sustained release of the model drug Rhodamine B from US-gel demonstrated its drug release capability. G. Cell-encapsulated US-gel was prepared by integrating cells into biocompatible US-ink and printing at a speed of 10 mm/min using an 8.75 MHz transducer at 7 W. H. Live/dead staining images observed the viability of C2C12 mouse myoblasts encapsulated in alginate US-gel on days 1 and 3 post-printing. Scale bar: 100 μm. I. From day 1 to day 7 post-printing, the metabolic activity of the cells was assessed. The illustration shows the cell-loaded US-gel pattern printed at a speed of 10 mm/min using an 8.75 MHz transducer, showing live cells after 3 days. Scale bar: 200 μm. J. The potential for bioadhesive applications was demonstrated by mixing catechol-modified gelatin-caffeic acid conjugate (GelCA) US-ink with NaIO4 liposomes. K. The adhesion strength of GelCA US-ink was tested before and after crosslinking. The illustration shows images of GelCA US-ink before and after slight heating and crosslinking. L. In ex vivo adhesion tests, GelCA US-gel was used to seal perforated cardiac tissue. Scale bar: 5 mm. M. In vivo US-induced adhesion, FUS facilitated the injection of prepolymers into tissues, followed by in situ crosslinking of alginate US-ink to achieve mechanical interlocking. N. In live animals, alginate US-gel was printed by intradermal injection of US-ink and crosslinked using a 2.65 MHz transducer at a speed of 20 mm/min, showing strong interfacial adhesion between alginate US-gel and tissues, with blue dye used for visibility. Scale bar: 6 mm. Conclusion: Figure 4 demonstrates the potential of ultrasound-based 3D printing technology in various medical applications of biomaterials, including conductivity, drug release, cell encapsulation, and bioadhesion.
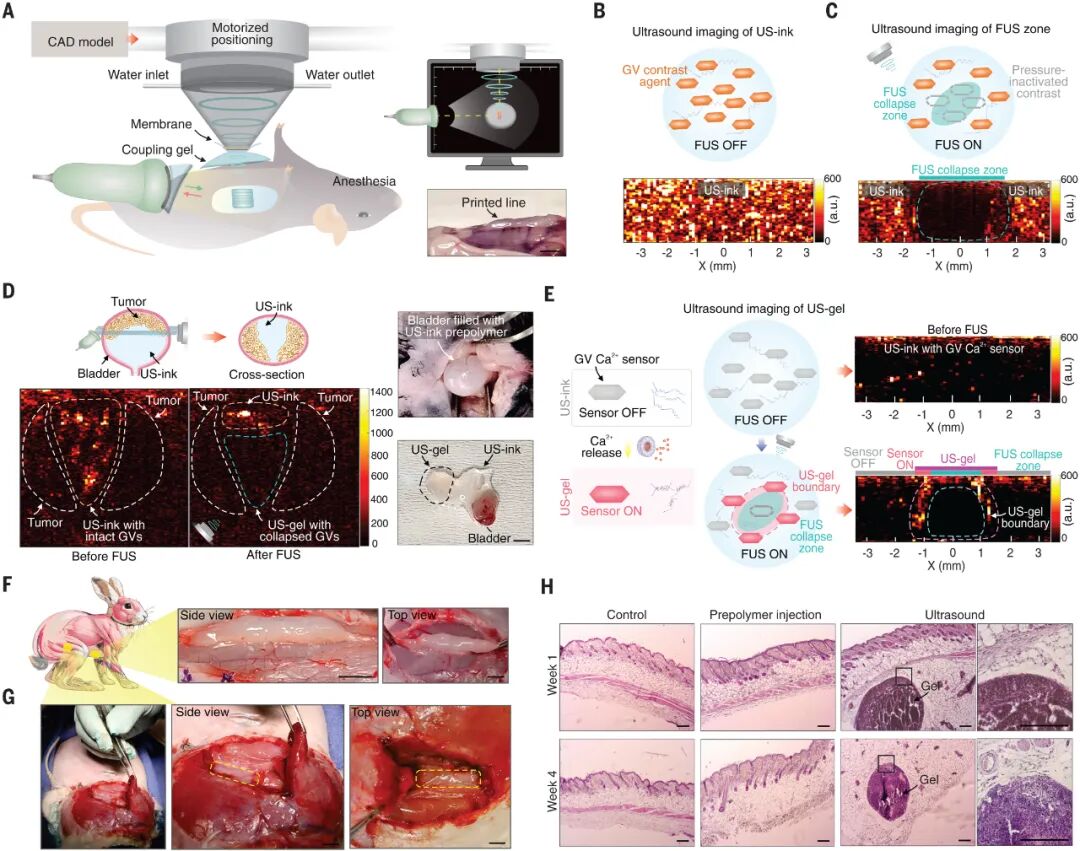
 Figure 5: Imaging-Guided In Vivo Deep Tissue Acoustic Printing
Figure 5: Imaging-Guided In Vivo Deep Tissue Acoustic Printing
Figure 5 illustrates the imaging-guided deep tissue acoustic printing technology and its experimental results in live animals. A. To perform acoustic printing in live animals, ultrasound imaging was used for precise localization. The illustration shows a linear pattern printed in vivo in a mouse, with a scale bar of 4 millimeters. B-C. AM mode ultrasound imaging and GV contrast agents were used to monitor the distribution of US-ink in vivo and ensure precise localization. The ultrasound image illustration shows the integration of GV with alginate US-ink printed in cross-section. GVs not exposed to focused ultrasound (FUS) remained intact, while those exposed to FUS collapsed. D. In vivo printing of US-gels at the site of bladder tumors in anesthetized mice. Successful localization was confirmed by GV collapse. After printing, the mouse bladder was extracted to verify printing success, with a scale bar of 4 millimeters. E. In situ Ca2+ sensing using GV Ca2+ sensors integrated into alginate US-inks, designed to activate upon exposure to Ca2+. AM mode ultrasound imaging printed and imaged a line in cross-section. Higher pressure at the center of the printed line caused some GV Ca2+ sensors to collapse, while those at the boundary of the printed US-gel were activated, confirming the shape. F. US-gel lines were printed in the deep adductor muscle and below the biceps femoris using 2.65 MHz FUS at 11 W and 15 mm/min, with a scale bar of 5 millimeters. G. US-gel lines were printed in the deep adductor muscle and below the biceps femoris using 2.65 MHz FUS at 20 W and 10 mm/min, with a scale bar of 5 millimeters. H. Biocompatibility of US-ink intradermal injection and ultrasound-printed US-gel in mice was assessed using hematoxylin-eosin staining (H&E), evaluated at 1 week and 4 weeks post-printing on skin tissues, with a scale bar of 200 micrometers. Conclusion: The experiments demonstrate the application of imaging-guided deep tissue acoustic printing technology in live animals, validating its effectiveness in precise localization, shape confirmation, and biocompatibility.
06

Main Conclusions
This paper by Davoodi et al. published in Science introduces an imaging-guided deep tissue in vivo acoustic printing (DISP) platform. This technology utilizes ultrasound to perform 3D bioprinting deep within the body, addressing the issue of invasive surgery required by traditional methods. DISP achieves precise and rapid crosslinking by integrating low-temperature sensitive liposomes carrying crosslinking agents into bio-inks. This method was validated in vivo in mouse bladders and rabbit leg muscles, demonstrating its potential in localized drug delivery and tissue replacement. DISP can print conductive, drug-loaded, cell-loaded, and bioadhesive biomaterials, showcasing its multifunctionality in various biomedical applications.
07

Discussion Summary
The study indicates that DISP technology achieves high precision and high-resolution deep in vivo printing of biological structures through imaging-guided ultrasound. By utilizing numerous crosslinking chemistries, this technology designs various bio-inks, including conductive, drug-loaded, cell-loaded, and bioadhesive formulations. Real-time ultrasound imaging ensures precise target localization and crosslinking control in vivo. Not only do in vitro studies show high biocompatibility, but in vivo studies also confirm the high biocompatibility of prepolymers and printed hydrogels. As a proof of concept, this technology successfully achieved in vivo printing in mouse bladders and rabbit leg muscles, demonstrating its potential in precise therapeutic interventions and tissue replacement. This innovation opens new possibilities for future precision medicine and tissue engineering. —END—
—END—