Following a series of DAC transactions last year, interest in this area has gradually increased, leading to various discussions and reviews. Among the products in the clinical phase of DAC, AbbVie‘s product ABBV-787 frequently appears alongside ORM-5029 and ORM-6151, but unlike the latter two, ABBV-787 has never disclosed any technical details in public forums. Although it is noted in the official NIH NCI Drug Dictionary that ABBV-787 is an anti-CD33/BET degrader ADC, this information was recorded later.
According to my somewhat vague impression, ABBV-787 was not even updated in AbbVie‘s pipeline for a period after it first came to public attention, with only NCT06068868 being a reliable source of clinical registration. It is speculated that the main reference for including it as a DAC in statistics was based on the inclusion and exclusion criteria (excluding patients who received CD33 targeted therapy within three months) and detection indicators (blood drug concentration of non-conjugated BET degrader).

Recently, I casually checked the progress of ABBV-787 and unexpectedly found that, according to an update on ClinicalTrials.gov on March 6, 2025, the status of NCT06068868 has been marked as Terminated, with the reason being Strategic consideration. As of now, no further information has been disclosed.

In December 2024, AbbVie publicly disclosed a related patent for a CD33-BET degrader ADC, which may be related to ABBV-787. Here is a brief analysis of some information from the abstract.

In the example of WO2024249235A1, AbbVie selected a different antibody from Gemtuzumab and employed a similar Thiomab site-specific conjugation method. Specifically, a cysteine site for conjugation was introduced at Y296C, and N297A was deglycosylated to reduce steric hindrance and improve conjugation efficiency. The site-specific conjugation at this position can utilize the hydrophobic effect of the original pocket to shield the linker-payload.
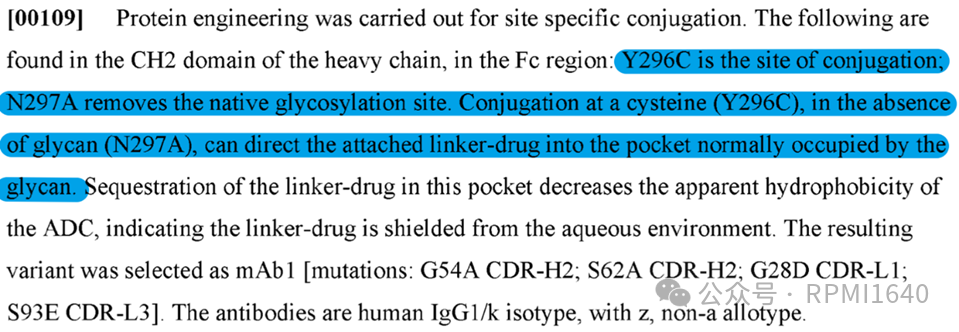
In terms of small molecule selection, a BET binder similar to GNE-987 and a VHL E3 ligase ligand were used, with the conjugation site on the BET binder, rather than on the hydroxyl-bearing VHL ligand. The cleavage fragment is Ala-Ala. There is no self-degradable fragment (spacer) directly conjugated to the aromatic amine, which is similar to AZ‘s camptothecin platform. The technology for the conjugation linker has built upon previous accumulations by AbbVie in the BCL-xL project, by introducing polar electron-withdrawing groups to induce the opening of the succinimide after conjugation, thereby avoiding the loss of the linker-payload due to reverse Michael addition.
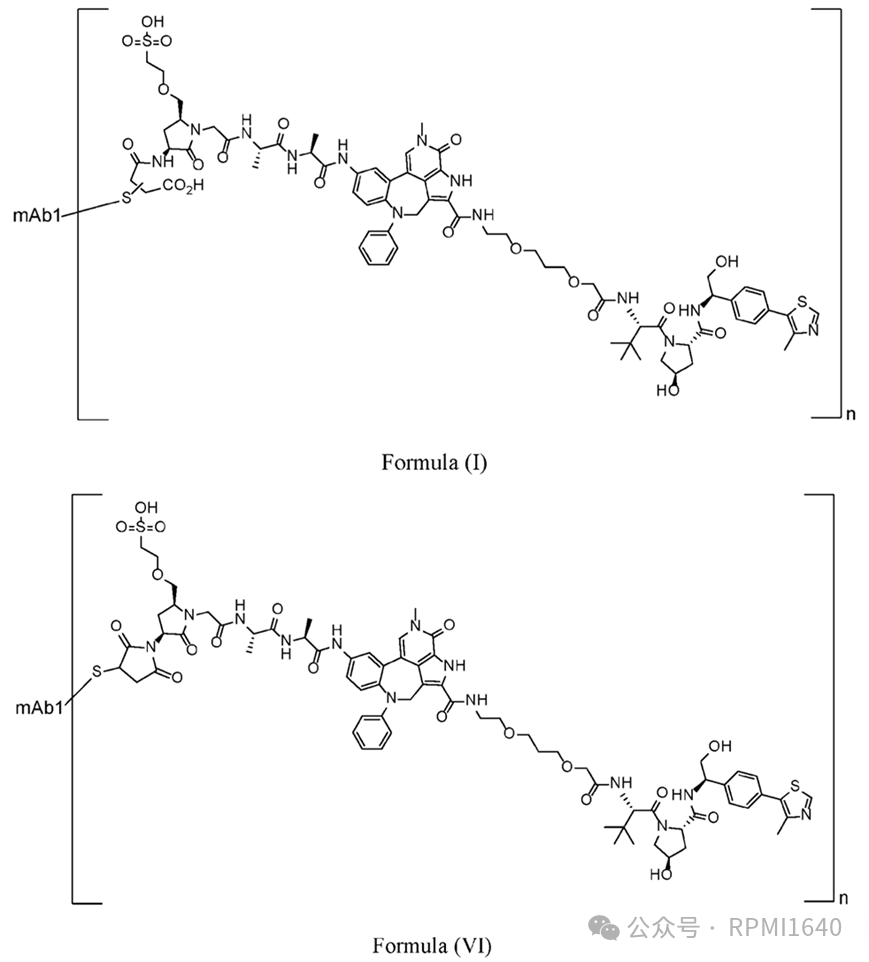
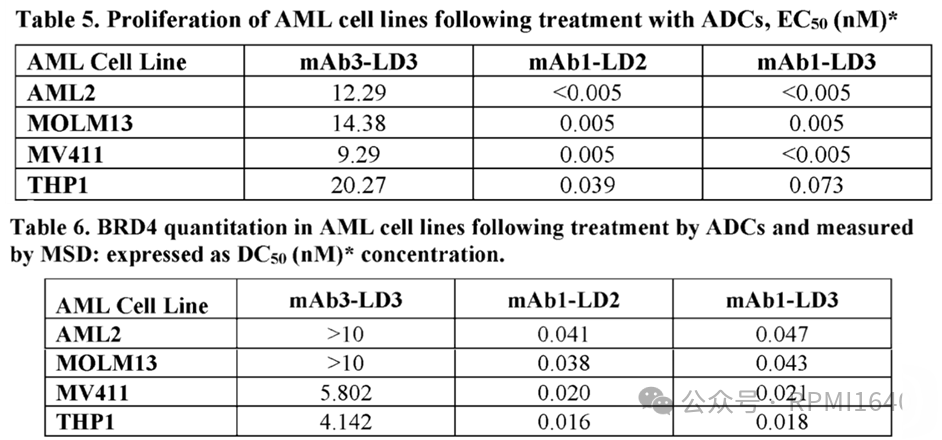
There are not many DAC constructs in the examples, with mAb1 targeting CD33 antibody, mAb3 being a negative control antibody, LD2 resembling the open-ring structure of Formula (I), and LD3 resembling the non-open-ring structure of Formula (VI). Since it is site-specific conjugation, the DAR is consistently 2. In vitro evaluations of the proliferation inhibition activity against AML cell lines and the degradation activity against BRD4 showed good performance for both mAb1-LD2 and mAb1-LD3, with good specificity, but no direct comparison was made with the degrader payload.
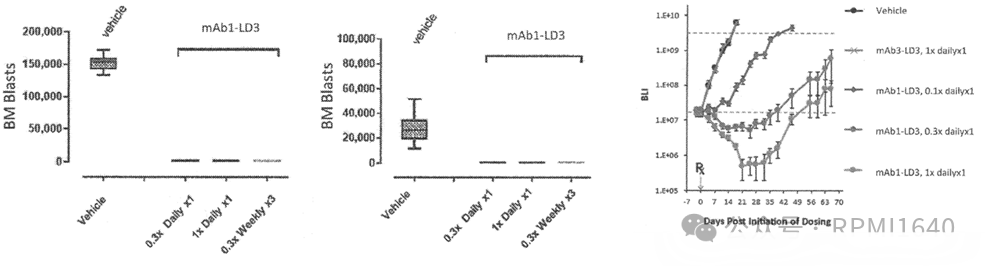
There are two in vivo examples, one PDX and one CDX, with the CDX appearing to be a mixed inoculation. The dosing of 1×, 0.3×, 0.1× seems a bit unclear; if it refers to mpk, the efficacy is good. Interestingly, the molecules used in the published data are all mAb1-LD3, which is the non-open-ring DAC.
Since Genentech began exploring BET DAC, and with two related publications from Eisai last year, there have been attempts by various MNCs and Biotechs to explore BET DAC. However, apart from ABBV-787, no other BET DAC has entered clinical trials, and the considerations involved may be more complex than anticipated.
Related References
https://clinicaltrials.gov/study/NCT06068868
WO2024249235A1
10.1126/sciadv.ado7120