

Characteristics: These nanosheets have a distinct ultrathin morphology, with an average thickness of approximately 0.9 nanometers, and exhibit short-range ordered structures characteristic of sub-crystalline materials.
02 Electrocatalytic Performance of FeCoP Catalyst
HER Performance: At 10 mA·cm-1, close to theoretical values, indicating that the HER process on the FeCoP catalyst undergoes a rapid Volmer-Heyrovsky pathway.
OER Performance: FeCoP shows good OER kinetics at 10 mA·cm-1.
03 Advantages of FeCoP Catalyst
High number of active sites: The ultrathin nanosheet structure of FeCoP increases the number of active sites, extending from the surface to the bulk phase, enhancing electrocatalytic performance.
High structural stability: The sub-crystalline structure maintains high structural stability effectively through short-range ordered arrangements, extending the catalyst’s lifespan.
Rapid charge transfer: FeCoP has a small charge transfer resistance (Rct), facilitating the rapid transfer of electrons during the catalytic process.
04 Catalytic Mechanism of FeCoP
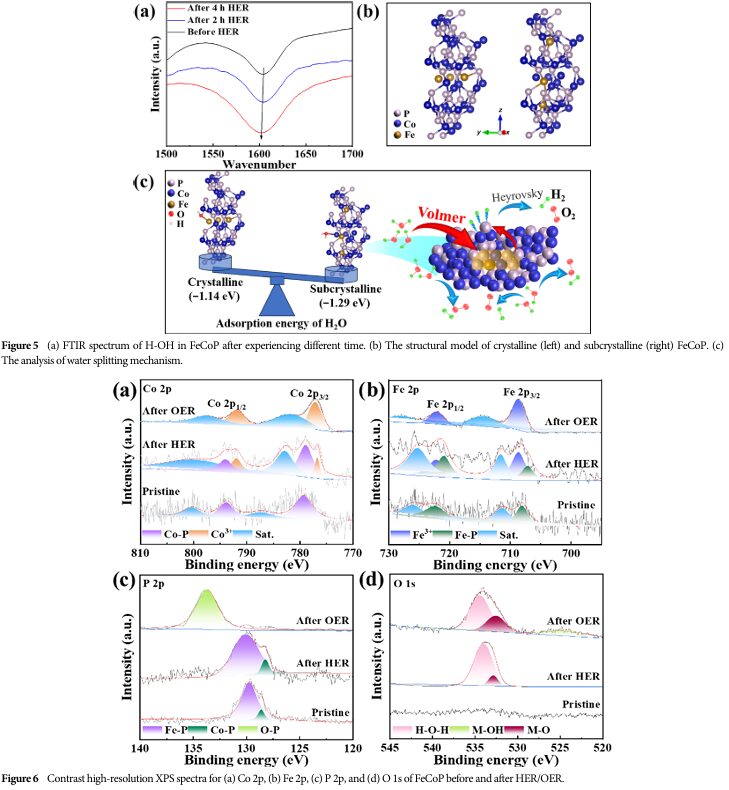
Theoretical calculations show that sub-crystalline FeCoP more easily adsorbs H2O molecules, thus accelerating the kinetics of the Volmer step in the HER process.
FTIR analysis indicates that during the water splitting process, the bending vibration peak of the O-H bond experiences a red shift, suggesting weakening of the O-H bond, providing sufficient H* species for subsequent Heyrovsky and Tafel steps.
05 Elemental Composition and Valence State Changes of FeCoP
XPS analysis reveals the elemental composition and valence state changes of FeCoP during the HER and OER processes.
After OER, only the characteristic peaks of oxidized Co3+ and Fe3+ are present, indicating that FeCoP is oxidized during the OER process.

06 Illustrated Guide
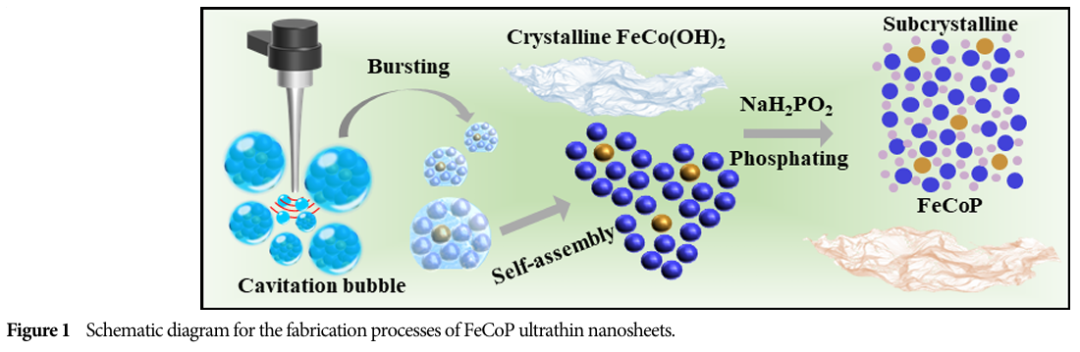
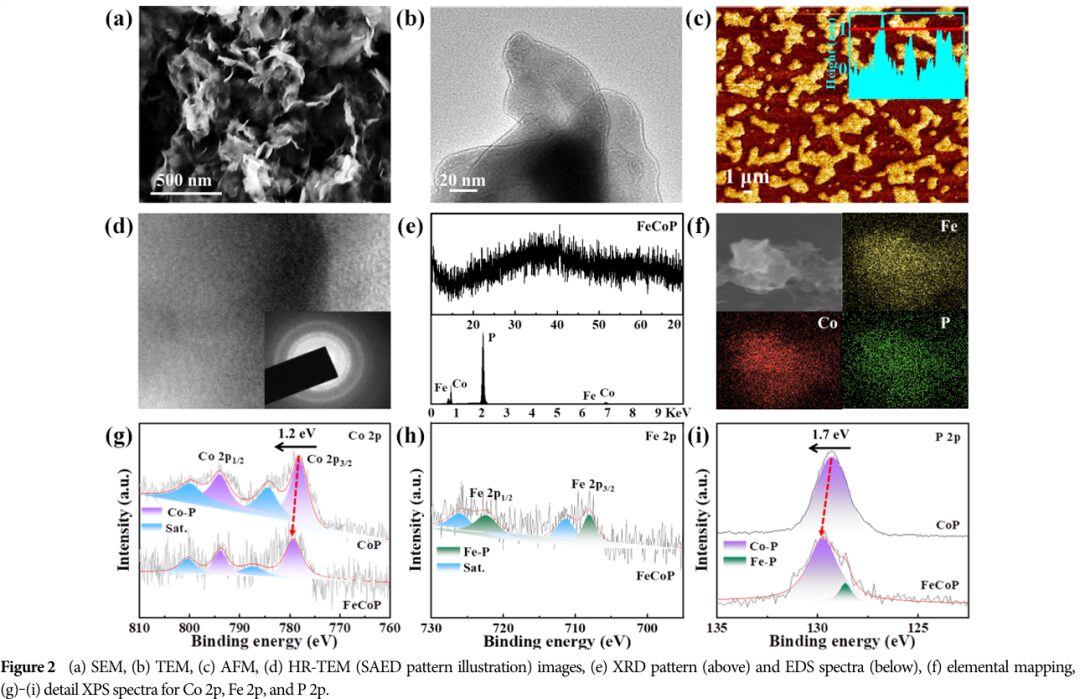
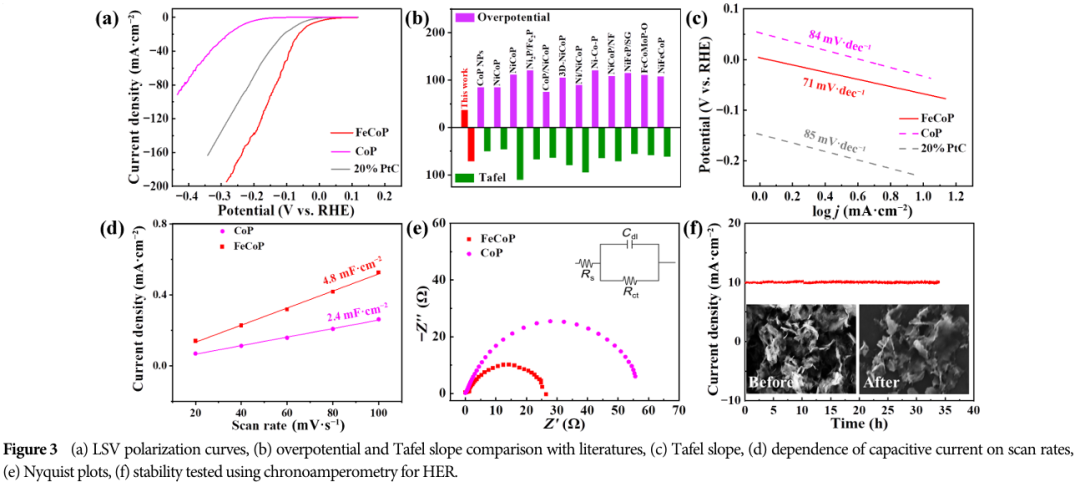
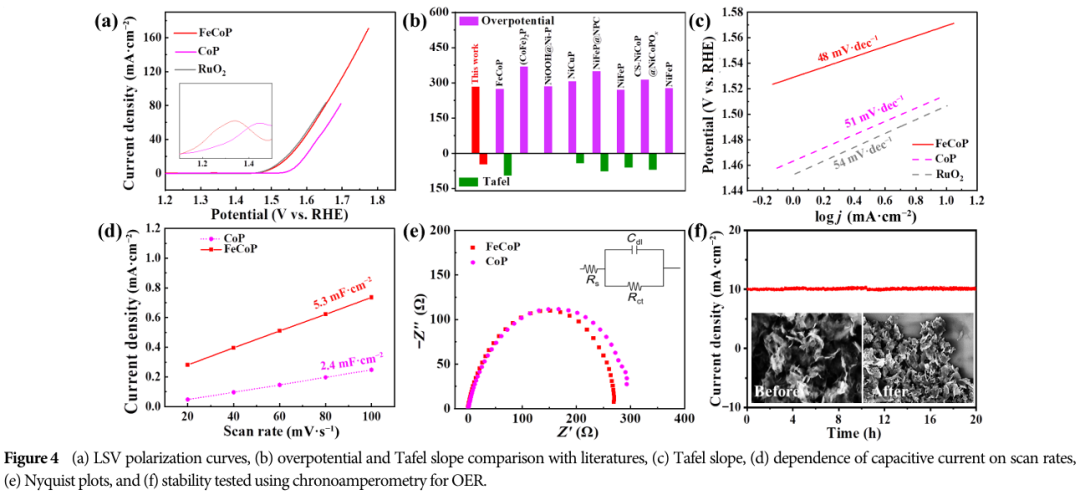
07 Practical Applications of FeCoP
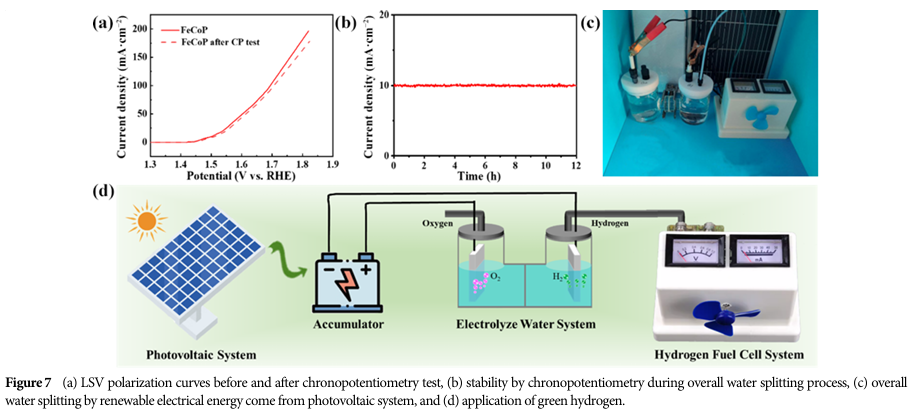
FeCoP, as a bifunctional catalyst, is used for overall water splitting.
When combined with solar photovoltaic systems, this system can utilize renewable energy to produce green hydrogen, which can be directly applied to hydrogen fuel cells to power a fan for over 10 hours.
08 Research Conclusions and Prospects
-
This study successfully constructs sub-crystalline FeCoP ultrathin nanosheets as efficient alkaline water splitting catalysts.
-
Its excellent HER and OER performance indicates that FeCoP has potential applications in commercial overall water splitting.
-
Future research can further explore the stability and durability of FeCoP catalysts, as well as composites with other materials to further enhance their electrocatalytic performance.
09 Summary
In summary, this article demonstrates efficient electrocatalysis for overall water splitting reactions by constructing sub-crystalline FeCoP ultrathin nanosheets, showcasing its tremendous potential in renewable energy conversion and storage.