“This article is for medical professionals only”
As the application of biologics in psoriasis continues to become widespread, have you had such experiences in your clinical work? “Early patients show significant clinical efficacy when using biologics, but over time, a certain proportion of patients always exhibit a decline in efficacy?” What is behind this? Let’s delve into this issue in this article, focusing on the immunogenicity of biologics and its clinical treatment impact.
Immunogenicity
The “Blockers” of Safety and Efficacy
Immunogenicity refers to the induction of anti-drug antibodies (ADAs) during the process of biotherapy 2, which can neutralize or alter the clearance of biologics, reducing clinical efficacy. Clearly, the immunogenicity of biologics is closely related to clinical treatment outcomes, with adverse outcomes ranging from mild, non-specific transient reactions to severe, life-threatening conditions (Figure 1) 3.
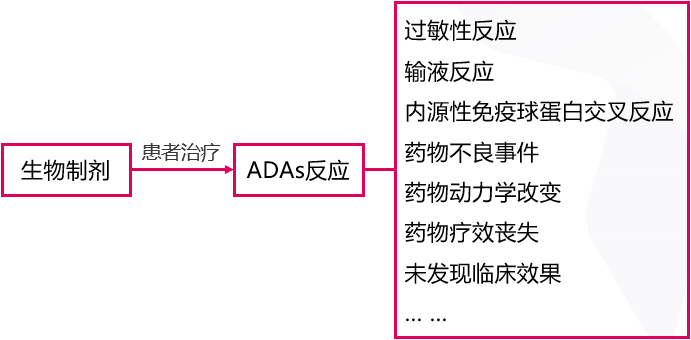
Figure 1. ADA responses in biotherapy may lead to changes in efficacy and potential serious side effects
When ADAs occur, infusion-related reactions, acute or non-acute allergic reactions, and other issues follow, directly or indirectly affecting drug safety and clinical efficacy, and even leading to treatment failure 3. Therefore, reducing immunogenicity is a key step in optimizing clinical treatment outcomes.
Research has found that almost all biologics used to treat psoriasis have ADAs 2. However, due to various influencing factors such as patients’ underlying diseases, immune status, age, genetic history, drug targets, and the characteristics of the drugs themselves 3, not all patients will produce ADA responses.
It is noteworthy that the frequency of ADA responses varies among different drugs, and the homology and targeting of monoclonal antibodies are key factors affecting immunogenicity 2,4.
Fully Human IL-17A
Rare Monoclonal Antibody
The era of monoclonal antibody therapy originated from the hybridoma technology for producing mouse monoclonal antibodies 5, but it has many limitations, mainly in immunogenicity, lack of effector function, and short half-life. The emergence of chimeric antibodies and humanized antibody technologies has reduced mouse components, greatly overcoming the shortcomings of mouse monoclonal antibodies. To this day, many antibody drugs are still chimeric or humanized antibodies from rodent origins (Figure 2) 5,7.
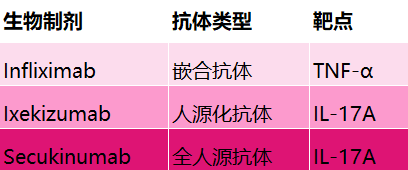
Figure 2. Types of antibodies and action targets for psoriasis biologics
With the continuous iteration and updating of research methods, phage display technology and the use of transgenic mice expressing human immunoglobulins have successfully created fully human antibodies (Figure 2), minimizing the immunogenicity of biologics (Figure 3) 5,6.
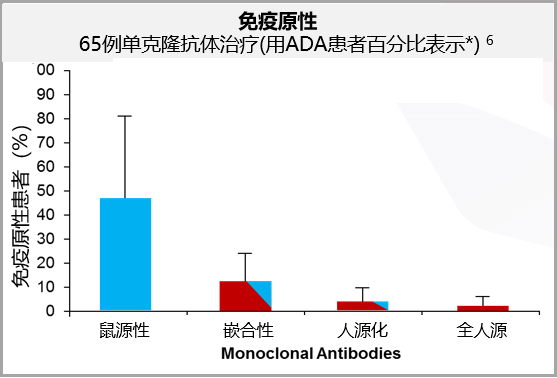
Figure 3. Clinical trial data of 65 monoclonal antibody treatments show that fully human antibodies have lower immunogenicity
Secukinumab, as the world’s first fully human IL-17A inhibitor, has also gained attention and recognition from psoriasis clinicians.
Clinical Data
Strength Against Immunogenicity
8
An analysis published in the British Journal of Dermatology (BJD) included 6 Phase III clinical trials involving 2842 subjects with moderate to severe plaque psoriasis undergoing secukinumab treatment for up to 52 weeks, assessing ADA during treatment. The results showed that only 11 subjects (0.4%) experienced ADA responses.
Conclusion

The efficacy and safety of biologic therapy for psoriasis have always been the focus of dermatologists and psoriasis patients, and the clinical significance of reducing the immunogenicity of biologics is already well understood.
The immunogenicity of biologics may not be completely avoided in the short term; how to select treatment medications and develop treatment plans to minimize it is a question that every clinician needs to ponder.Fully human monoclonal antibodies may be one of the key factors in ensuring efficacy and safety, but with the goal of comprehensively optimizing clinical treatment outcomes and helping patients achieve a higher quality of life, researchers still have a long way to go.
References:
1. J Caleb, S Sahil, Y Di, et al. Monoclonal antibodies inhibiting IL-12, -23, and -17 for the treatment of psoriasis. HUMAN VACCINES & IMMUNOTHERAPEUTICS. 2017, 13(10); 2247–2259.
2. J Denis, P Jorg C., N Frank O. Immunogenicity of Biotherapy Used in Psoriasis: The Science Behind the Scenes. Journal of Investigative Dermatology. 2015, 135; 31-38.
3. J Eva-Maria, S Christian K. How to systematically evaluate immunogenicity of therapeutic proteins–regulatory considerations. New Biotechnology. 2009, 25(5); 280-286.
4. Van Walle I et al. Drug Discovery World. 2006; Summer: 94;
5. C Andrew C., C Paul J. Therapeutic antibodies for autoimmunity and inflammation. Immunology. 2010, (10); 301-316.
6. G Sean H, H Kexin, T Hua, et al. Monoclonal antibody humanness score and its applications. BMC Biotechnology. 2013, 13:55. https://bmcbiotechnol.biomedcentral.com/articles/10.1186/1472-6750-13-55.
7. G Paolo, G Davide, P Miriam, et al. State of the art and pharmacological pipeline of biologics for chronic plaque psoriasis. Current Opinion in Pharmacology. 2019, 46: 90–99.
8. K. Reich, A. Blauvelt, A. Armstrong, et al. Secukinumab, a Fully Human Anti–Interleukin-17A Monoclonal Antibody, Exhibits Minimal Immunogenicity in Subjects with Moderate to Severe Plaque Psoriasis. British Journal of Dermatology. 2017; 176: 752.
9. D Thaçi, et al. P1994, presented at 27th EADV, September 2018, Paris.
10. K. Reich, A. Blauvelt, A. Armstrong, et al. Secukinumab, a fully human anti-interleukin-17A monoclonal antibody, exhibits low immunogenicity in psoriasis patients over 5 years.
Cover image: Zcool Hairo
Accompanying images: Dingxiangyuan
Editor: Wen Yaxue
Source: Skin Time
If you find this article useful, please share it with more friends.
Finally, please press and hold the QR code below to follow the Center for Skin Prevention, and every morning at 7 am, lock in here to learn more skin knowledge!