IntroductionAll-solid-state lithium batteries (ASSLBs) are considered a key energy storage technology with great development prospects due to their high safety and energy density. As the core component of all-solid-state lithium batteries, the development of solid electrolytes has attracted significant attention from both academia and industry. Currently, solid electrolytes are mainly divided into two categories: inorganic solid electrolytes represented by oxides and sulfides, and organic solid electrolytes represented by polyethylene oxide (PEO). Given the great potential of composite solid electrolytes in combining the advantages of both types, extensive research has been conducted in recent years.Recently, Professor Chen Zhongwei from the University of Waterloo systematically summarized and analyzed the basic situation of composite solid-state electrolytes (CSSEs) for lithium batteries, including an overview of early development history, basic introduction to ionic transport mechanisms, as well as research progress in key materials, advanced structures, in situ testing methods, and applications of artificial intelligence/machine learning. The author first categorized the materials and structures of organic/inorganic CSSEs into four major types: inorganic material filling, layered structures, three-dimensional continuous structures, and open framework structures, providing an in-depth analysis of the structural design strategies and ionic transport mechanisms involved. Finally, the author summarized the main challenges faced by CSSEs and proposed future development directions. The related results were published in the internationally renowned journal Chemical Society Reviews under the title A review of composite solid-state electrolytes for lithium batteries: fundamentals, key materials and advanced structures, with the first author being postdoctoral researcher Zheng Yun from the University of Waterloo.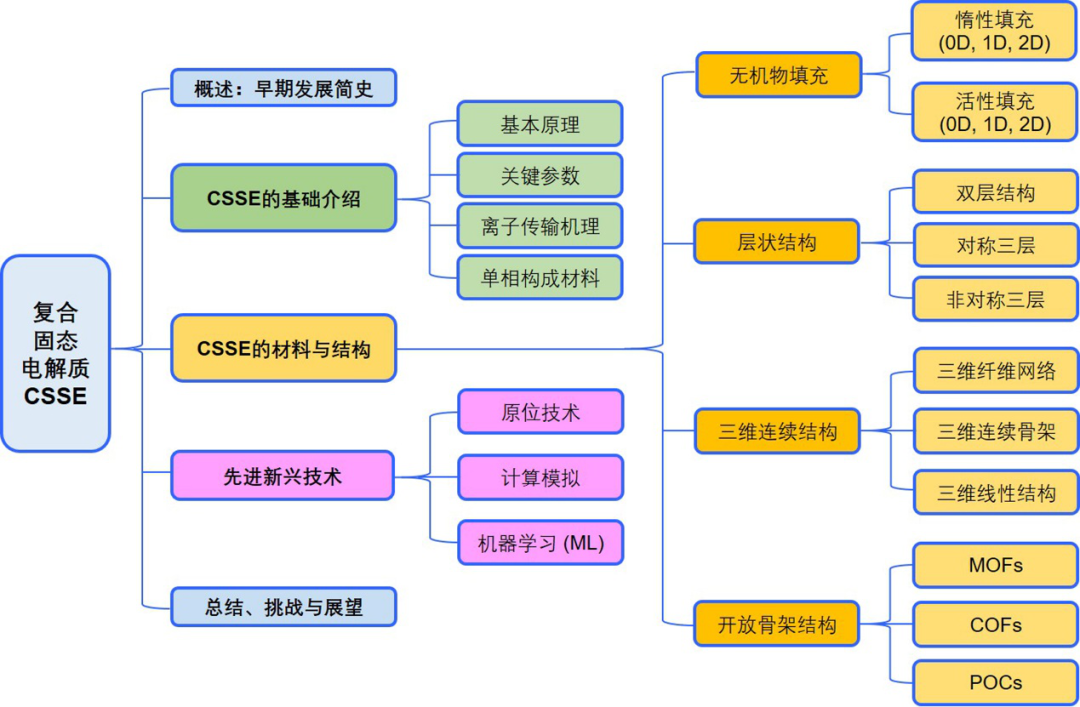 Overview of the Review (Article Outline)Visual Guide1. Basic IntroductionThe basic principles and main structures of all-solid-state lithium batteries are shown in Figure 1, which mainly consist of a negative electrode represented by metallic lithium, a positive electrode represented by materials such as LFP (LiFePO4) and NMC (LiNixMnyCo(1-x-y)O2), and a solid electrolyte. The key evaluation indicators for the performance of solid electrolytes mainly include ionic conductivity, lithium ion transference number, mechanical properties, electrochemical stability, and battery testing performance. The main single-phase materials include perovskite-type, garnet-type, NASICON-type, LISICON-type, sulfide-type, and polymer-type. The corresponding lithium ion transport mechanisms mainly involve vacancy mechanisms, interstitial mechanisms, or free volume models.This article mainly focuses on organic/inorganic composite solid electrolytes, which can be roughly divided into three categories based on the combination method, as shown in the figure: filling inorganic components into organic solids (Inorganic fillers in the polymer matrix), organic/inorganic bilayer or multilayer structures (Heterogeneous layered structure), and filling organic components into three-dimensional continuous inorganic structures (3D inorganic continuous framework with filled polymer); among them, composite solid electrolytes related to open framework materials such as MOFs, COFs, and POCs mainly belong to the first category.
Overview of the Review (Article Outline)Visual Guide1. Basic IntroductionThe basic principles and main structures of all-solid-state lithium batteries are shown in Figure 1, which mainly consist of a negative electrode represented by metallic lithium, a positive electrode represented by materials such as LFP (LiFePO4) and NMC (LiNixMnyCo(1-x-y)O2), and a solid electrolyte. The key evaluation indicators for the performance of solid electrolytes mainly include ionic conductivity, lithium ion transference number, mechanical properties, electrochemical stability, and battery testing performance. The main single-phase materials include perovskite-type, garnet-type, NASICON-type, LISICON-type, sulfide-type, and polymer-type. The corresponding lithium ion transport mechanisms mainly involve vacancy mechanisms, interstitial mechanisms, or free volume models.This article mainly focuses on organic/inorganic composite solid electrolytes, which can be roughly divided into three categories based on the combination method, as shown in the figure: filling inorganic components into organic solids (Inorganic fillers in the polymer matrix), organic/inorganic bilayer or multilayer structures (Heterogeneous layered structure), and filling organic components into three-dimensional continuous inorganic structures (3D inorganic continuous framework with filled polymer); among them, composite solid electrolytes related to open framework materials such as MOFs, COFs, and POCs mainly belong to the first category.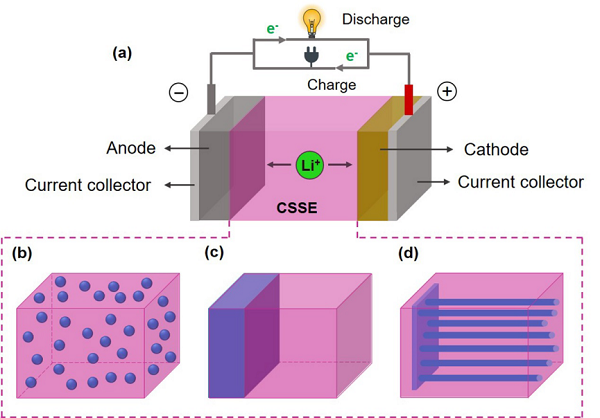 Figure 1. Three Typical Combination Methods of Lithium Composite Solid-State Electrolytes2. Materials and StructuresThe inorganic materials involved in the first combination method mainly include inert metal oxides/non-metal oxides and several active single-phase inorganic solid electrolyte materials, where the judgment of inertness and activity is based on whether they can effectively conduct lithium ions; the organic part mainly consists of polymers represented by PEO (containing lithium salts). Depending on the characteristics of the filling materials, the article summarizes them into 0D, 1D, and 2D, corresponding to nanoparticles, nanowires/rods, and two-dimensional sheet materials, respectively. The ionic conductivity of the obtained CSSEs at room temperature (10–6-10–3 S cm-1) can be improved by several times to over a hundred times compared to single-phase materials; among them, the performance enhancement mechanisms of inert (TiO2, SiO2, ZrO2, BaTiO3, SrBi4Ti4O15, CNT, MMT, LDH, etc.) or active inorganic fillers (LLZO, LLTO, LATP, etc.) differ, which the article analyzes in detail. Figures 2 and 3 show some research examples.
Figure 1. Three Typical Combination Methods of Lithium Composite Solid-State Electrolytes2. Materials and StructuresThe inorganic materials involved in the first combination method mainly include inert metal oxides/non-metal oxides and several active single-phase inorganic solid electrolyte materials, where the judgment of inertness and activity is based on whether they can effectively conduct lithium ions; the organic part mainly consists of polymers represented by PEO (containing lithium salts). Depending on the characteristics of the filling materials, the article summarizes them into 0D, 1D, and 2D, corresponding to nanoparticles, nanowires/rods, and two-dimensional sheet materials, respectively. The ionic conductivity of the obtained CSSEs at room temperature (10–6-10–3 S cm-1) can be improved by several times to over a hundred times compared to single-phase materials; among them, the performance enhancement mechanisms of inert (TiO2, SiO2, ZrO2, BaTiO3, SrBi4Ti4O15, CNT, MMT, LDH, etc.) or active inorganic fillers (LLZO, LLTO, LATP, etc.) differ, which the article analyzes in detail. Figures 2 and 3 show some research examples.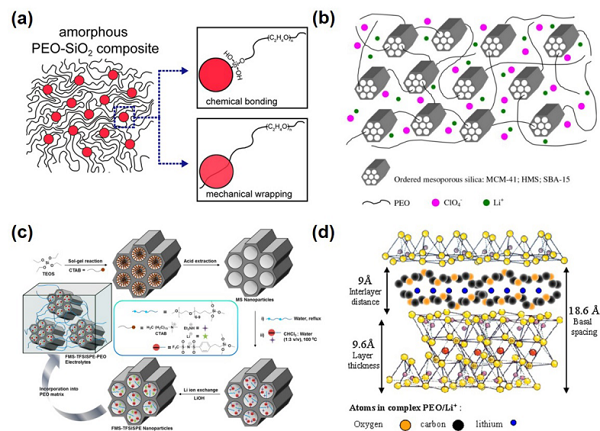 Figure 2. Zero-Dimensional (0D) Inert Inorganic Material Filled in Polymer to Form Composite Solid Electrolyte
Figure 2. Zero-Dimensional (0D) Inert Inorganic Material Filled in Polymer to Form Composite Solid Electrolyte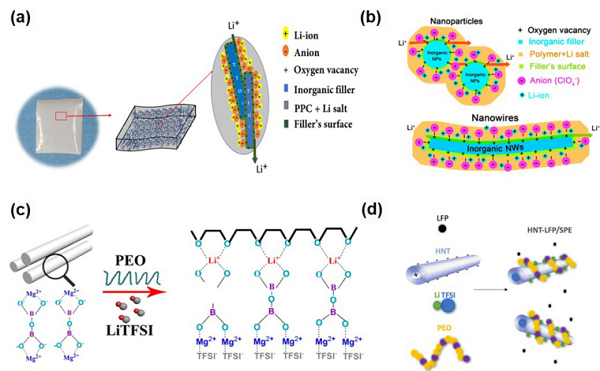 Figure 3. One-Dimensional (1D) Inert Inorganic Material Filled in Polymer to Form Composite Solid ElectrolyteThe second combination method is heterogeneous organic/inorganic layered, mainly including double-layer (Double-layered architecture), symmetrical three-layer (Symmetrical sandwiched architecture), and asymmetrical three-layer (Asymmetric sandwiched architecture). Among them, organic layers such as PEO, PEGDA, PAN, PMA can significantly improve the contact between the inorganic layer and the electrode, and even enhance the electrochemical stability of the electrolyte by improving its oxidation or reduction resistance; while inorganic layers such as LAGP, LLZO can improve the ionic conductivity and mechanical strength of the composite electrolyte. A few case studies are shown in Figure 4, with more related content detailed in the article.
Figure 3. One-Dimensional (1D) Inert Inorganic Material Filled in Polymer to Form Composite Solid ElectrolyteThe second combination method is heterogeneous organic/inorganic layered, mainly including double-layer (Double-layered architecture), symmetrical three-layer (Symmetrical sandwiched architecture), and asymmetrical three-layer (Asymmetric sandwiched architecture). Among them, organic layers such as PEO, PEGDA, PAN, PMA can significantly improve the contact between the inorganic layer and the electrode, and even enhance the electrochemical stability of the electrolyte by improving its oxidation or reduction resistance; while inorganic layers such as LAGP, LLZO can improve the ionic conductivity and mechanical strength of the composite electrolyte. A few case studies are shown in Figure 4, with more related content detailed in the article.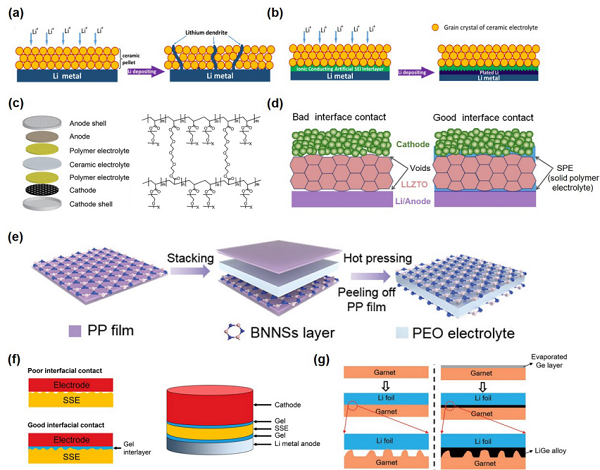 Figure 4. Double-layer or Three-layer Composite Solid ElectrolyteThe third combination method involves filling organic components into inorganic three-dimensional continuous structures. Based on the characteristics of the inorganic three-dimensional continuous structures, this article roughly divides them into three types: (1) three-dimensional fiber networks (3D interconnected fiber network), (2) three-dimensional continuous frameworks (3D continuous framework), and (3) vertically aligned three-dimensional structures (Vertically aligned 3D framework), with specific structural characteristics shown in Figures 5 and 6. Unlike the mixing of 0D to 2D inorganic materials with organic polymers in the first category, three-dimensional continuous structures can effectively reduce or even avoid interfacial resistance between inorganic lithium ion conductor particles, thereby improving the transport capability of lithium ions. Compared to the first two types of three-dimensional continuous structures, the third type can further optimize the transport path of lithium ions and avoid “detours”. Detailed case analyses and mechanism introductions are also covered in the original text.
Figure 4. Double-layer or Three-layer Composite Solid ElectrolyteThe third combination method involves filling organic components into inorganic three-dimensional continuous structures. Based on the characteristics of the inorganic three-dimensional continuous structures, this article roughly divides them into three types: (1) three-dimensional fiber networks (3D interconnected fiber network), (2) three-dimensional continuous frameworks (3D continuous framework), and (3) vertically aligned three-dimensional structures (Vertically aligned 3D framework), with specific structural characteristics shown in Figures 5 and 6. Unlike the mixing of 0D to 2D inorganic materials with organic polymers in the first category, three-dimensional continuous structures can effectively reduce or even avoid interfacial resistance between inorganic lithium ion conductor particles, thereby improving the transport capability of lithium ions. Compared to the first two types of three-dimensional continuous structures, the third type can further optimize the transport path of lithium ions and avoid “detours”. Detailed case analyses and mechanism introductions are also covered in the original text.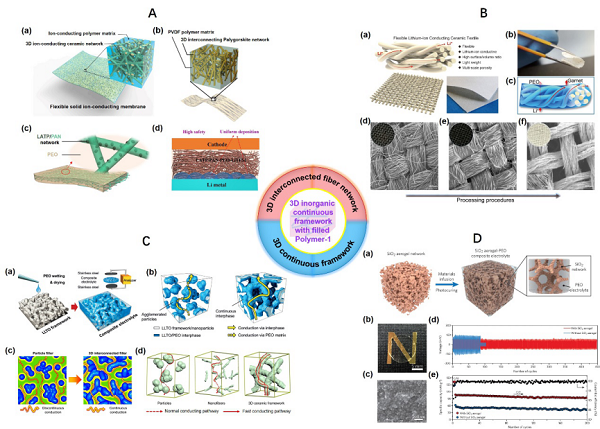 Figure 5. Composite Solid Electrolyte Containing Three-Dimensional Fiber Network and Three-Dimensional Continuous Framework
Figure 5. Composite Solid Electrolyte Containing Three-Dimensional Fiber Network and Three-Dimensional Continuous Framework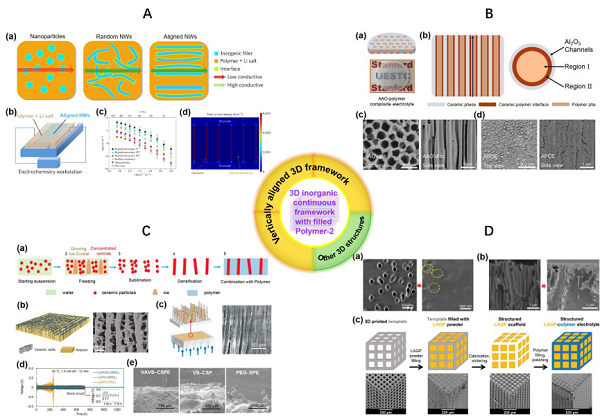 Figure 6. Composite Solid Electrolyte Containing Three-Dimensional Linear Continuous StructureThe last category involves three recently popular open framework structure materials, which are porous materials with high porosity (>90%), large internal surface area (6000 m2 g−1), designability, adjustable pore sizes, and easy functionalization of pore surfaces, widely studied and applied in drug delivery, gas separation, energy conversion, and other fields. Considering their specific characteristics, this article summarizes and analyzes solid/composite solid electrolytes related to these materials in a dedicated section, specifically including solid electrolytes related to MOFs (Metal–organic frameworks, Figure 7), COFs (Covalent organic frameworks, Figure 8), and POCs (Porous organic cages, Figure 9). Research shows that the addition of these materials can lead to an increase in ionic conductivity of solid electrolytes by one to two orders of magnitude. Detailed performance comparisons and optimization mechanism analyses are also summarized in the original text.
Figure 6. Composite Solid Electrolyte Containing Three-Dimensional Linear Continuous StructureThe last category involves three recently popular open framework structure materials, which are porous materials with high porosity (>90%), large internal surface area (6000 m2 g−1), designability, adjustable pore sizes, and easy functionalization of pore surfaces, widely studied and applied in drug delivery, gas separation, energy conversion, and other fields. Considering their specific characteristics, this article summarizes and analyzes solid/composite solid electrolytes related to these materials in a dedicated section, specifically including solid electrolytes related to MOFs (Metal–organic frameworks, Figure 7), COFs (Covalent organic frameworks, Figure 8), and POCs (Porous organic cages, Figure 9). Research shows that the addition of these materials can lead to an increase in ionic conductivity of solid electrolytes by one to two orders of magnitude. Detailed performance comparisons and optimization mechanism analyses are also summarized in the original text.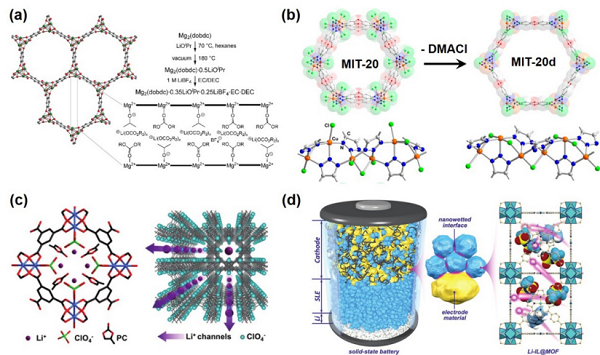 Figure 7. Research on Composite Solid Electrolytes Related to MOFs
Figure 7. Research on Composite Solid Electrolytes Related to MOFs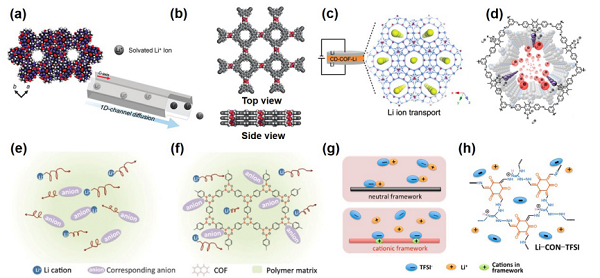 Figure 8. Research on Composite Solid Electrolytes Related to COFs
Figure 8. Research on Composite Solid Electrolytes Related to COFs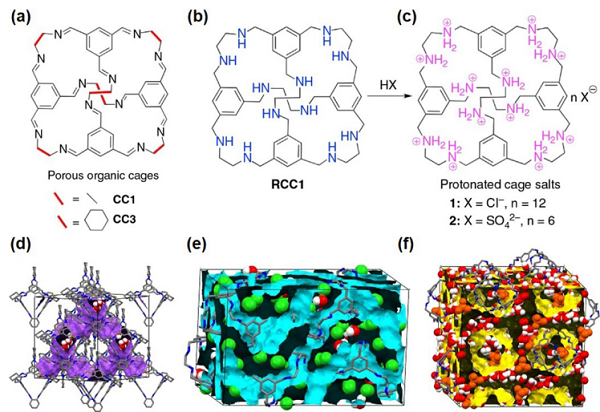 Figure 9. Research on Composite Solid Electrolytes Related to POCs3. Advanced Emerging TechnologiesThis article also summarizes advanced emerging technologies specifically for composite solid electrolytes, including (1) targeted in situ characterization techniques, (2) emerging simulation technologies, and (3) the very popular applications of artificial intelligence/machine learning in composite solid electrolytes.Among them, in situ NDP, solid-state NMR, and operando XTM characterization methods have unique advantages in studying solid ionic transport mechanisms, solid electrolyte interfaces (SEI), lithium dendrites, and other issues. Emerging simulation technologies such as ab initio molecular dynamics (AIMD, Figure 10) are more suitable than traditional DFT and MD for exploring lithium ion transport paths in solid materials, thereby optimizing material screening and design.As an important branch of artificial intelligence (AI), machine learning (ML) is currently applied in solid/composite solid electrolytes mainly in four aspects: material screening, structural performance exploration, ionic transport mechanism investigation, and optimization of the composition of composite solid electrolytes. Specific case studies are shown in Figure 11, with detailed summaries and analyses in the original text.
Figure 9. Research on Composite Solid Electrolytes Related to POCs3. Advanced Emerging TechnologiesThis article also summarizes advanced emerging technologies specifically for composite solid electrolytes, including (1) targeted in situ characterization techniques, (2) emerging simulation technologies, and (3) the very popular applications of artificial intelligence/machine learning in composite solid electrolytes.Among them, in situ NDP, solid-state NMR, and operando XTM characterization methods have unique advantages in studying solid ionic transport mechanisms, solid electrolyte interfaces (SEI), lithium dendrites, and other issues. Emerging simulation technologies such as ab initio molecular dynamics (AIMD, Figure 10) are more suitable than traditional DFT and MD for exploring lithium ion transport paths in solid materials, thereby optimizing material screening and design.As an important branch of artificial intelligence (AI), machine learning (ML) is currently applied in solid/composite solid electrolytes mainly in four aspects: material screening, structural performance exploration, ionic transport mechanism investigation, and optimization of the composition of composite solid electrolytes. Specific case studies are shown in Figure 11, with detailed summaries and analyses in the original text.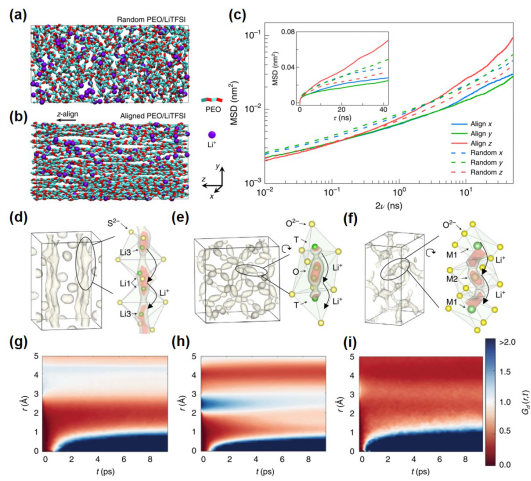 Figure 10. Application of Ab Initio Molecular Dynamics (AIMD) in Composite Solid Electrolytes
Figure 10. Application of Ab Initio Molecular Dynamics (AIMD) in Composite Solid Electrolytes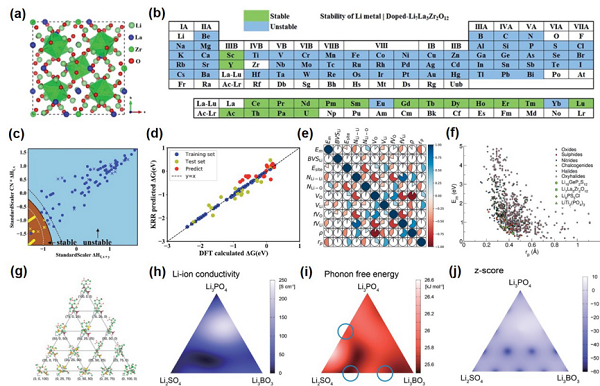 Figure 11. Application of Artificial Intelligence/Machine Learning (AI/ML) in Composite Solid ElectrolytesConclusion and OutlookThis article provides a systematic and comprehensive summary and analysis of the research on composite solid-state electrolytes (CSSEs) from four aspects: development history, basic introduction, materials and structures, and advanced emerging technologies. In particular, it systematically categorizes and details the key materials and advanced structures of CSSEs from four aspects. Based on this, the article also summarizes the main challenges currently faced in this field and proposes four targeted strategies to address them: (1) in-depth research on lithium ion conduction mechanisms and material behaviors in CSSEs; (2) developing new materials/optimizing structures to improve the ionic conductivity of CSSEs; (3) optimizing and enhancing the stability of CSSEs under high voltage and wide temperature ranges; (4) improving the technological maturity and economic feasibility of all-solid-state lithium batteries containing CSSEs to promote their further practical applications.Reference link: “Yun Zheng et al., A review of composite solid-state electrolytes for lithium batteries: fundamentals, key materials and advanced structures” (Chemical Society Reviews, 2020, 473, 228607, https://doi.org/10.1039/D0CS00305K).Research Group IntroductionChen Zhongwei, Professor in the Department of Chemical Engineering at the University of Waterloo, Fellow of the Royal Society of Canada, Fellow of the Canadian Academy of Engineering, Canada’s National Chief Scientist (CRC-Tier 1), Vice President of the International Academy of Electrochemical Energy Science, and Director of the Electrochemical Energy Center at the University of Waterloo, serves as the associate editor of ACS Applied & Material Interfaces. Professor Chen leads a research team of about 70 people dedicated to the development of advanced materials and electrodes for sustainable energy systems, including fuel cells, metal-air batteries, lithium-ion batteries, lithium-sulfur batteries, flow batteries, solid-state batteries, CO2 capture and conversion, etc. In recent years, he has published over 330 papers in top international journals such as Nature Energy, Nature Nanotechnology, Chemical Reviews, Chemical Society Reviews, Joule, Matter, Nature Communication, Journal of the American Chemical Society, Angewandte Chemie International Edition, Advanced Materials, Energy & Environmental Science, ACS Nano, with over 29,000 citations and an H-index of 87.Research Group Homepage:http://chemeng.uwaterloo.ca/zchen/This article is from Materials People, submitted by author Zheng Yun.
Figure 11. Application of Artificial Intelligence/Machine Learning (AI/ML) in Composite Solid ElectrolytesConclusion and OutlookThis article provides a systematic and comprehensive summary and analysis of the research on composite solid-state electrolytes (CSSEs) from four aspects: development history, basic introduction, materials and structures, and advanced emerging technologies. In particular, it systematically categorizes and details the key materials and advanced structures of CSSEs from four aspects. Based on this, the article also summarizes the main challenges currently faced in this field and proposes four targeted strategies to address them: (1) in-depth research on lithium ion conduction mechanisms and material behaviors in CSSEs; (2) developing new materials/optimizing structures to improve the ionic conductivity of CSSEs; (3) optimizing and enhancing the stability of CSSEs under high voltage and wide temperature ranges; (4) improving the technological maturity and economic feasibility of all-solid-state lithium batteries containing CSSEs to promote their further practical applications.Reference link: “Yun Zheng et al., A review of composite solid-state electrolytes for lithium batteries: fundamentals, key materials and advanced structures” (Chemical Society Reviews, 2020, 473, 228607, https://doi.org/10.1039/D0CS00305K).Research Group IntroductionChen Zhongwei, Professor in the Department of Chemical Engineering at the University of Waterloo, Fellow of the Royal Society of Canada, Fellow of the Canadian Academy of Engineering, Canada’s National Chief Scientist (CRC-Tier 1), Vice President of the International Academy of Electrochemical Energy Science, and Director of the Electrochemical Energy Center at the University of Waterloo, serves as the associate editor of ACS Applied & Material Interfaces. Professor Chen leads a research team of about 70 people dedicated to the development of advanced materials and electrodes for sustainable energy systems, including fuel cells, metal-air batteries, lithium-ion batteries, lithium-sulfur batteries, flow batteries, solid-state batteries, CO2 capture and conversion, etc. In recent years, he has published over 330 papers in top international journals such as Nature Energy, Nature Nanotechnology, Chemical Reviews, Chemical Society Reviews, Joule, Matter, Nature Communication, Journal of the American Chemical Society, Angewandte Chemie International Edition, Advanced Materials, Energy & Environmental Science, ACS Nano, with over 29,000 citations and an H-index of 87.Research Group Homepage:http://chemeng.uwaterloo.ca/zchen/This article is from Materials People, submitted by author Zheng Yun.
Note: Images are sourced from the internet; please notify for removal if there are any copyright issues!